As filed with the Securities and Exchange Commission on November 8, 2022.
Registration No. 333-267392
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________
Amendment No. 5
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
____________________
ASP Isotopes Inc.
(Exact name of Registrant as specified in its charter)
____________________
|
Delaware |
2890 |
87-2618235 |
||
|
(State or other jurisdiction of |
(Primary Standard Industrial |
(I.R.S. Employer |
433 Plaza Real, Suite 275
Boca Raton, Florida 33432
(561) 709-3034
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
____________________
Paul E. Mann
Chairman and Chief Executive Officer
ASP Isotopes Inc.
433 Plaza Real, Suite 275
Boca Raton, Florida 33432
(561) 709-3034
(Name, address, including zip code, and telephone number, including area code, of agent for service)
____________________
Copies to:
|
Brenda Hamilton, Esq. Boca Raton, Fl 33432 |
Ross D. Carmel, Esq. |
____________________
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated file |
☐ |
Accelerated filer |
☐ |
|||||
|
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|||||
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion, Dated November 8, 2022
1,500,000 Shares
ASP Isotopes Inc.
Common Stock
____________________
This is the initial public offering of shares of common stock of ASP Isotopes Inc. We are offering 1,500,000 shares of common stock.
The selling stockholders identified in this prospectus are offering an additional 2,057,500 shares of our common stock, which we refer to as the “selling stockholder shares.” We will not receive any proceeds from the sale of selling stockholder shares. The selling stockholder shares will not be purchased by the underwriters or otherwise included in the underwritten offering of our common stock in this initial public offering. The selling stockholders may sell or otherwise dispose of their shares in a number of different ways and at varying prices, but will not sell any selling stockholder shares until after the closing of this offering. See “Selling Stockholders — Plan of Distribution.” We will pay all expenses (other than discounts, concessions, commissions and similar selling expenses, if any) relating to the registration of the selling stockholders’ shares of common stock with the Securities and Exchange Commission.
No public market currently exists for our common stock. We have applied to list our common stock on the Nasdaq Capital Market under the symbol “ASPI.” The closing of this offering is contingent upon the successful listing of our Common Stock on the Nasdaq Capital Market.
We anticipate that the initial public offering price per share will be between $4.00 and $5.00.
We are an “emerging growth company” as defined under the federal securities laws, and as such, we have elected to comply with certain reduced reporting requirements for this prospectus and may elect to do so in future filings.
Investing in our common stock involves risks. See “Risk Factors” beginning on page 9 of this prospectus.
____________________
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
____________________
|
Per Share |
Total |
|||||
|
Initial public offering price |
$ |
|
$ |
|
||
|
Underwriting discount(1) |
$ |
|
$ |
|
||
|
Proceeds, before expenses, to ASP Isotopes Inc.(1) |
$ |
|
$ |
|
||
____________
(1) We have agreed to pay Revere Securities LLC, as representative of the underwriters named in this prospectus, or the Representative, a discount equal to 7.0% of the gross proceeds of the offering. See the section titled “Underwriting” for additional information regarding underwriting discounts, commissions and estimated offering expenses.
To the extent that the underwriters sell more than 1,500,000 shares of common stock, the underwriters have a 45-day option to purchase up to an additional 225,000 shares of common stock from us at the initial public offering price less the underwriting discount.
____________________
The underwriters expect to deliver the shares against payment in New York, New York on , 2022.
Sole Book Running Manager
Revere Securities LLC
____________________
Prospectus dated , 2022.
|
Page |
||
|
1 |
||
|
9 |
||
|
43 |
||
|
45 |
||
|
46 |
||
|
47 |
||
|
48 |
||
|
50 |
||
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
52 |
|
|
64 |
||
|
83 |
||
|
89 |
||
|
97 |
||
|
101 |
||
|
103 |
||
|
107 |
||
|
112 |
||
|
Material U.S. Federal Income Tax Considerations to Non-U.S. Holders |
114 |
|
|
118 |
||
|
122 |
||
|
122 |
||
|
122 |
||
|
F-1 |
____________________
You should rely only on the information contained in this prospectus and any free writing prospectus that we may provide to you in connection with this offering. Neither we, the selling stockholders nor the underwriters have authorized anyone to provide you with different information or to make any other representations, and neither we, the selling stockholders nor the underwriters take responsibility for, and can provide no assurance as to the reliability of, any other information others may give you. We and the selling stockholders are offering to sell, and seeking offers to buy, shares of our common stock only under circumstances and in jurisdictions where it is lawful to do so. Neither we, the selling stockholders nor any of the underwriters are making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than its date. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: Neither we, the selling stockholders nor any of the underwriters have done anything that would permit this offering or the possession or distribution of this prospectus in any jurisdiction where action for those purposes is required, other than in the United States. Persons outside of the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of our common stock and the distribution of this prospectus outside of the United States.
i
This summary highlights selected information that is presented in greater detail elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our common stock. You should read this entire prospectus carefully, including the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our consolidated financial statements and the related notes included elsewhere in this prospectus before making an investment decision. Unless the context otherwise requires, the terms “Company,” “we,” “us,” “our” or similar terms refer to ASP Isotopes Inc. together with its consolidated subsidiaries. References to the “selling stockholders” refer to the selling stockholders named in this prospectus.
The Company
We are a pre-commercial stage advanced materials company dedicated to the development of technology and processes that, if successful, will allow for the production of isotopes that may be used in several industries. We have an exclusive license to use proprietary technology, the Aerodynamic Separation Process (“ASP technology”), originally developed and licensed to us by Klydon Proprietary Ltd (“Klydon”), for the production, distribution, marketing and sale of all isotopes. Our initial focus is on the production and commercialization of enriched Molybdenum-100 (“Mo-100”). Klydon has agreed to provide us a first commercial-scale Mo-100 enrichment plant located in South Africa with a manufacturing capacity of 20 kg/year of 95% enriched Mo-100 when fully operational. We believe that the Mo-100 we may develop using the ASP technology has significant potential advantages for use in the preparation of nuclear imaging agents by radiopharmacies and others in the medical industry. We also intend to use the ASP technology licensed to us by Klydon to produce enriched Uranium-235 (“U-235”). We believe that the U-235 we may develop using the ASP technology may be commercialized as a nuclear fuel component for use in the new generation of HALEU-fueled small modular reactors that are now under development for commercial and government uses.
We may also seek to use the ASP technology to separate Silicon-28, which we believe has potential application in the quantum computing target end market, and Carbon-14, which we believe has potential application in the pharma/agrochem target end market. In addition, we are considering future development of the ASP technology for the separation of Zinc-68, Ytterbium-176, Zinc-67, Nickel-64 and Xenon-136 for potential use in the healthcare target end market, and Chlorine -37 and Lithium-6 for potential use in the nuclear energy target end market.
We operate principally through subsidiaries: ASP Isotopes Guernsey Limited (the holding company of ASP Isotopes South Africa (Proprietary) Limited), which will be focused on the development and commercialization of high value, low volume isotopes for highly specialized end markets (such as Mo-100 and others, including Silicon-28); Enriched Energy LLC, which will be focused on the development and commercialization of uranium for the nuclear energy market; and ASP Isotopes UK Ltd, which is the licensee of the ASP technology under the exclusive license agreement with Klydon.
Background
We were incorporated in Delaware in September 2021 to acquire assets and license intellectual property rights related to the production of Mo-100 using the ASP technology. The aerodynamic separation technique has its origins in the South African uranium enrichment program in the 1980s and the ASP technology has been developed during the last 18 years by the scientists at Klydon. In Klydon’s testing, the ASP technology has demonstrated efficacy and commercial scalability in the enrichment of oxygen-18 and silicon-28. In July 2022, ASP Isotopes UK Ltd, as licensee, entered into a license agreement with Klydon, as licensor, pursuant to which ASP Isotopes UK Ltd acquired from Klydon an exclusive license to use, develop, modify, improve, subcontract and sublicense certain intellectual property rights relating to the ASP technology for the production, distribution, marketing and sale of all isotopes produced using the ASP technology (the “Klydon license agreement”). The Klydon license agreement is royalty-free, has a term of 999 years and the license is worldwide for the development of the ASP technology and the distribution, marketing and sale of isotopes. Future production of isotopes is limited to member countries of the Nuclear Suppliers Group. We have no products approved for commercial sale or existing customers and have generated no revenues to date. We have not yet built a functioning Mo-100 or U-235 enrichment plant or even demonstrated the ability to produce Mo-100 or U-235 using the ASP technology.
1
Dr Einar Ronander, who serves as Chief Scientific Adviser to our board of directors, and Dr Hendrik Strydom, one of our directors, previously co-founded and currently serve as Executive Chairperson and Chief Executive Officer, of Klydon. Dr Ronander and Dr Strydom are the controlling shareholders of Klydon through Isotope Separation Technology (Pty) Ltd, a company jointly owned by Dr Ronander and Dr Strydom and the largest shareholder of Klydon. Dr Ronander and Dr Strydom each own approximately 11.9% of our outstanding shares of common stock. Immediately following the closing of this offering, Dr Ronander and Dr Strydom will each own 11.2% of our outstanding shares of common stock (or approximately 11.1% of our common stock, if the underwriters exercise in full their option to purchase additional shares of our common stock in this offering). For more information on our transactions with Klydon, see the section of this prospectus entitled “Certain Relationships and Related Party Transactions — Our Relationship with Klydon Proprietary Limited”).
Our Strategy
Our goal is to develop technology and processes that, if successful, will allow for the production of isotopes that may be used in the medical isotopes, nuclear power or other industries. Key elements of our strategy to achieve this goal include:
• Complete development and commissioning of Mo-100 enrichment facility located in Pretoria, South Africa.
• Demonstrate the capability to produce Mo-100 using the ASP technology and capitalize on the opportunity to solve the Mo-99 supply chain challenges in the existing medical isotope market.
• Introduce Mo-100 produced using ASP technology as an alternative and potentially more convenient production route for Tc-99m.
• Explore commercial opportunities for Silicon-28 and other light isotopes that may be produced using the assets comprising a dormant Silicon-28 aerodynamic separation processing plant acquired from Klydon in July 2022 (which are also located in Pretoria, South Africa).
• Continue identifying potential offtake customers and strategic partners for Mo-100. We expect limited commercial activity for Mo-100 in the United States during the next two to three years and we anticipate that most of our initial revenues from future sales of our Mo-100 will be derived from countries in Asia and EMEA (Europe, Middle East and Africa).
• Demonstrate the capability to produce high-assay low-enriched uranium (HALEU) using the ASP technology and meet anticipated demand for the new generation of HALEU-fueled small modular reactors and advanced reactor designs that are now under development for commercial and government uses.
Mo-100 Regulatory Approvals
We have not sought any regulatory approval for the application of Mo-100. Currently, the sale or use of Mo-100 is not regulated by a healthcare regulator, such as the FDA, European Medicines Agency (EMA) or comparable foreign regulatory authorities. However, products such as Mo-99 and Tc-99m that are produced from Mo-100 in a cyclotron or a linear accelerator are regulated by healthcare regulators. Our future customers who may use Mo-100 to produce radiopharmaceuticals will likely require regulatory approval for their products. To date, only one healthcare regulator (Canada) has approved the use of Tc-99m produced from Mo-100 via a cyclotron. Obtaining regulatory approval is expensive and can take many years to complete, and its outcome is inherently uncertain. Our customers’ regulatory approval process may not be conducted as planned or completed on schedule, if at all, and failure can occur at any time during the process.
Intellectual Property
We have an exclusive worldwide license to use, develop, modify, improve, subcontract and sublicense certain intellectual property rights relating to the ASP technology for the production, distribution, marketing and sale of all isotopes produced using the ASP technology. The intellectual property rights granted to us through the Klydon license agreement include all existing and/or future proprietary rights of Klydon relating to the ASP technology, whether or not such rights have been registered including the copyright, designs, know-how, patents and trademarks (although
2
Klydon currently has no such patents, patent applications or copyrights). Klydon has spent the last 18 years and tens of millions of dollars developing the aerodynamic separation technique used in the ASP technology, generating critical trade secrets. Neither we nor Klydon have any existing patents, pending patent applications or copyrights. To date, we and Klydon have relied exclusively on trade secrets and other intellectual property laws, non-disclosure agreements with our respective employees, consultants, vendors, potential customers and other relevant persons and other measures to protect our intellectual property, and intend to continue to rely on these and other means.
Recent Developments
On October 25, 2022, we received a letter (the “NMS Letter”) from a law firm acting on behalf of Norsk Medisinsk Syklotronsenter AS (“NMS”), asserting, among other things, that the grant of a license to the ASP technology to ASP Isotopes Inc. by Klydon violates a pre-existing exclusive sub-license to the ASP technology for the separation of isotopes of Molybdenum that have medical applications granted to Radfarma, as more fully described below. NMS and ASP Isotopes had previously been briefly in discussions regarding a potential future collaboration on technology and product development. However, these preliminary discussions have not been active for several months, after the death of a lead NMS scientist and ASP Isotopes Inc.’s decision to explore other options.
The NMS Letter makes reference to: (1) a license agreement entered into on October 25, 2013 by Klydon and API Labs Pharmaceuticals (Proprietary) Limited (“API Labs”) to license the ASP technology for enriching certain isotopes of the element Molybdenum (“2013 API Labs License”); and (2) an exclusive sub-license to the ASP technology granted on October 1, 2019 to Radfarma, as licensee, by API Labs and SaPhotonica Limited (“SaPhotonica”), as licensors (the “2019 Radfarma Sub-License”). The NMS Letter states that Radfarma is a joint venture that is 45% owned by NMS and 45% owned by SaPhotonica.
The NMS Letter asserts, among other things, that the grant of a license to the ASP technology to ASP Isotopes Inc. by Klydon violates a covenant in the 2019 Radfarma Sub-License that the licensors shall not be entitled, directly or indirectly, to use, grant or otherwise give the rights, or any similar rights, which were granted to Radfarma under the 2019 Radfarma Sub-License to any other person for use in the territory. The sub-license granted to Radfarmain the 2019 RadfarmaSub-License related to the use of intellectual property rights related to the ASP technology for the separation of isotopes of Molybdenum that have medical applications. “Territory” is defined in the 2019 Radfarma Sub-License as “the Kingdom of Norway for the construction of the 20-kilogram capacity plants; and means the international market where distribution agreements can be produced.” The NMS Letter asserts that while Klydon purported to give to ASP Isotopes Inc. a license to market the ASP technology globally, these rights were already granted to Radfarma.
The NMS Letter includes a request for ASP Isotopes Inc. to enter into discussions for an agreement with NMS based on terms proposed in previous correspondence from NMS. The previous correspondence from NMS included the following key prerequisites to a possible cooperation between NMS and ASP Isotopes Inc.:(1) NMS will be granted the right to set up an enrichment facility for a Mo-100 in Norway; (2) NMS will be granted the exclusive rights to sales, marketing, and distribution in Europe, while ASPI gets similar rights for the rest of the world; and (3) NMS will support the development of the Mo-100 target production and Mo-99 generator production and if required the sales operations of ASPI.
The NMS Letter does not include a threat of litigation against ASP Isotopes Inc. or any parties to the 2013 API Labs License or 2019 Radfarma Sub-License. However, if the licensed rights granted to us are found to be invalid or unenforceable (in whole or in part), or if our exclusive license agreement with Klydon is terminated or Klydon, as licensor, fails to abide by the terms of our exclusive license agreement, our ability to commercialize our future isotopes would suffer and our business, results of operations and financial condition may be adversely affected. If the prior sub-license granted to Radfarmais found to be valid, we would be required to cease using the ASP technology for the separation of isotopes of Molybdenum that have medical applications (unless we were able to obtain a license from Radfarma), and we would focus our business operations on the enrichment of isotopes other than Molybdenum. For example, instead of continuing to pursue the production of Molybdenum-100, we could focus on the production and commercialization of zinc, silicon and/or chlorine using the ASP technology. We expect that our Mo-100 plant in South Africa would need to be redesigned and retrofitted in order to produce other isotopes, which would take approximately six months and cost approximately $1 million. See “Risk Factors — Risks Related to Our Intellectual Property” — “We have received a letter asserting that the license for the ASP technology granted to us from Klydon, which is critical to our business, may be invalid because these rights were already granted to a third party, Radfarma” and “Our license for the ASP technology with Klydon may be found to infringe third party intellectual property rights.”
3
Based on information currently available, and after consultation with legal counsel in South Africa, management believes that our exclusive license for the enrichment of Molybdenum-100 and all other isotopes from Klydon are valid and the company will vigorously defend its rights.
Summary Risk Factors
Investing in our common stock involves substantial risks, which are discussed more fully under the heading “Risk Factors” immediately following this summary. You should carefully consider all the information in this prospectus, including under “Risk Factors,” before making an investment decision. The risks described under the heading “Risk Factors” may cause us to not realize the full benefits of our strengths or may cause us to be unable to successfully execute all or part of our strategy. Some of the more significant challenges include the following:
• We have a very limited operating history, and we have incurred losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. We may never generate any revenue or become profitable or, if we achieve profitability, we may not be able to sustain it.
• The report of our independent registered public accounting firm for the period from September 13, 2021 (inception) through December 31, 2021 contains an explanatory paragraph that expresses substantial doubt about our ability to continue as a “going concern.”
• Even if this offering is successful, we will require substantial additional capital to finance our operations, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate certain of our development efforts or other operations.
• To become and remain profitable, we must succeed in developing and eventually commercializing isotopes that generate significant revenue. This will require us to be successful in a range of challenging activities, including completing research and development activities relating to our ASP technology, obtaining applicable regulatory approval for future isotopes, if any, and manufacturing, marketing and selling any future isotopes. We are only in the process of completing research and development activities relating to our ASP technology.
• Because we have targeted both Mo-100 and U-235 for production using the ASP technology, we may expend our limited resources to pursue a particular isotope and fail to capitalize on another isotope that may be more profitable or for which there is a greater likelihood of commercial success.
• We face substantial competition, which may result in others developing or commercializing isotopes before or more successfully than us.
• The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if our customers are ultimately unable to obtain regulatory approval for our Mo-100, our business will be substantially harmed.
• Klydon currently performs or supports many of our development activities and will continue to do so after the closing of this offering pursuant to our turnkey agreement, and if we are unable to replicate or replace these functions if this services agreement is terminated, our operations could be adversely affected.
• Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
• We have identified a material weakness in our internal control over financial reporting, and if our remediation of this material weakness is not effective, or if we experience material weaknesses in the future or otherwise fail to implement and maintain an effective system of internal controls in the future, we may not be able to accurately report our financial condition or results of operations which may adversely affect investor confidence in us, and as a result, the value of our common stock.
• Sales of a substantial number of shares of our common stock by our existing stockholders, including the selling stockholders, in the public market, or the perception that such sales could occur, could cause our stock price to fall.
4
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of relief from certain reporting requirements and other burdens that are otherwise applicable generally to public companies. These provisions include:
• reduced obligations with respect to financial data, including presenting only two years of audited financial statements and only two years of selected financial data;
• an exemption from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act;
• reduced disclosure about our executive compensation arrangements in our periodic reports, proxy statements, and registration statements; and
• exemptions from the requirements of holding non-binding advisory votes on executive compensation or golden parachute arrangements.
In addition, under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to avail ourselves of this exemption from new or revised accounting standards, and, therefore, we will not be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies or that have opted out of using such extended transition period, which may make comparison of our financial statements with those of other public companies more difficult. We may take advantage of these reporting exemptions until we no longer qualify as an emerging growth company, or, with respect to adoption of certain new or revised accounting standards, until we irrevocably elect to opt out of using the extended transition period.
We will remain an emerging growth company until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; and (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC. We may choose to take advantage of some but not all of these reduced reporting burdens.
Corporate Information
We were incorporated in Delaware in September 2021. Our principal executive offices are located at 433 Plaza Real, Suite 275, Boca Raton, Florida 33432, and our telephone number is (561) 709-3034. Our website address is www.aspisotopes.com. Information contained on, or that can be accessed through, our website is not part of and is not incorporated by reference into this prospectus, and you should not consider information on our website to be part of this prospectus.
5
The Offering
|
Common stock offered by us |
1,500,000 shares |
|
|
Selling stockholder shares |
2,057,500 shares |
|
|
Common stock outstanding after this offering |
|
|
|
Option to purchase additional shares of common stock offered in this offering |
|
|
|
Use of proceeds |
We estimate that the net proceeds to us from the sale of shares of our common stock in this offering will be approximately $5.4 million (or approximately $6.3 million if the underwriters’ option to purchase additional shares is exercised in full) based upon the assumed initial public offering price of $4.5 per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discount and estimated offering expenses payable by us. We will not receive any of the proceeds from the sale of selling stockholder shares. |
|
|
The principal purposes of this offering are to increase our capitalization and financial flexibility, create a public market for our common stock and thereby enable access to the public equity markets for us and our stockholders. We initiated Phase 1 of the Mo-100 development plan under the turnkey contract, targeting 5 kg/year of 95% enriched Mo-100, in October 2021 and we expect to complete this phase using cash on hand during the second half of 2022. Upon completion of Phase 1, we intend to use a portion of the net proceeds from this offering (currently estimated to be approximately $6.0 million of the total net proceeds) to initiate and fully fund Phase 2 of the Mo-100 development plan under the turnkey contract, which targets expanded production of up to 20 kg/year of 95% enriched Mo-100. We intend to use the remainder of the net proceeds we receive from this offering for research and development for potential additional isotopes that we may offer, as well as headcount costs, working capital and other for general corporate purposes. See “Use of Proceeds” for a more complete description of the intended use of proceeds from this offering. |
||
|
Voting rights |
Shares of our common stock are entitled to one vote per share. |
|
|
Underwriter compensation |
In connection with this offering, the underwriters will receive an underwriting discount equal to 7.0% of the offering price of the shares of common stock in the offering. In addition, we have agreed to: (i) reimburse the Representative for fees and expenses of legal counsel and other out-of-pocket expenses incurred in connection with this offering up to $150,000, plus closing costs not to exceed $12,900; (ii) reimburse the Representative for certain additional accountable expenses of the representative; (iii) provide the Representative with “tail” financing compensation under certain circumstances; and (iv) indemnify the underwriters for certain liabilities in connection with this offering. See “Underwriting”. |
6
|
Concentration of Ownership |
Upon the completion of this offering, our executive officers and directors, and their affiliates, will beneficially own, in the aggregate, approximately 34.1% of our outstanding shares of common stock, representing approximately 34.1% of the voting power of our outstanding shares of common stock (based on shares of common stock outstanding as of September 30, 2022 and excluding awards of 3,000,000 shares of restricted stock that we anticipate making upon the effectiveness of the registration statement of which this prospectus is a part). |
|
|
Risk factors |
You should read the section entitled “Risk Factors” and the other information included elsewhere in this prospectus for a discussion of some of the risks and uncertainties you should carefully consider before deciding to invest in our common stock. |
|
|
Dividend policy |
We currently do not intend to declare any dividends on our common stock in the foreseeable future. Our ability to pay dividends on our common stock may be limited by the terms of any future debt or preferred securities we may issue or any future credit facilities we may enter into. See the section titled “Dividend Policy.” |
|
|
Proposed Nasdaq Capital Market trading symbol |
|
The total number of shares of our common stock that will be outstanding after this offering is based on 30,107,127 shares of common stock outstanding as of September 30, 2022 and excludes:
• 2,901,000 shares of our common stock issuable upon the exercise of options outstanding as of September 30, 2022 under our 2021 Stock Incentive Plan, or the 2021 Plan, at a weighted-average exercise price of $1.91 per share; and
• 5,000,000 shares of common stock reserved for future issuance under our 2022 Equity Incentive Plan, or the 2022 Plan, under which we expect to make all future awards (including awards to our executive officers, directors and consultants of 3,000,000 shares of restricted stock that we anticipate making upon the effectiveness of the registration statement of which this prospectus is a part); and
• 57,250 shares of common stock issuable to Revere Securities LLC as partial compensation for its role as placement agent in connection with an unregistered offering of shares of common stock.
Unless otherwise indicated, this prospectus assumes or gives effect to the following:
• the filing and effectiveness of our amended and restated certificate of incorporation to be effective immediately prior to the closing of this offering, or our Certificate of Incorporation, and the adoption of our amended and restated bylaws to be effective immediately prior to the closing of this offering, or our Bylaws; and
• no exercise by the underwriters of their option to purchase 225,000 additional shares of our common stock.
Summary Consolidated Financial Data
The following tables present our summary consolidated financial data as of and for the period indicated. The statement of operations and comprehensive loss data for the six-month period ended June 30, 2022, and the balance sheet data as of June 30, 2022, are derived from our unaudited financial statements that are included elsewhere in this prospectus.
You should read this data together with our audited consolidated financial statements and related notes, as well as the information under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” included elsewhere in this prospectus. The summary financial data in this section are not intended to replace our financial statements and the related notes and are qualified in their entirety by the financial statements and related notes included elsewhere in this prospectus. Our historical results are not necessarily indicative of results that should be expected in any future period.
7
|
For the |
||||
|
Consolidated Statement of Operations Data: |
|
|
||
|
Operating expenses: |
|
|
||
|
Research and development |
|
446,440 |
|
|
|
General and administrative |
|
1,239,772 |
|
|
|
Total operating expenses |
|
1,686,212 |
|
|
|
|
|
|||
|
Other income |
|
|
||
|
Interest income |
|
1,454 |
|
|
|
Total other income |
|
1,454 |
|
|
|
|
|
|||
|
Net loss |
|
(1,684,758 |
) |
|
|
|
|
|||
|
Other comprehensive loss: |
|
|
||
|
Foreign currency translation |
|
125,271 |
|
|
|
Total comprehensive loss |
|
(1,559,487 |
) |
|
|
Net loss per share attributable to common stockholders, basic and diluted |
$ |
(0.06 |
) |
|
|
Weighted average common shares outstanding, basic and diluted |
|
27,898,098 |
|
|
|
As of June 30, |
||||||
|
|
Pro Forma, As |
|||||
|
(unaudited) |
||||||
|
Balance Sheet Data: |
|
|
||||
|
Cash |
$ |
2,813,411 |
$ |
8,320,321 |
||
|
Working capital(3) |
$ |
1,741,235 |
$ |
7,093,735 |
||
|
Total assets |
$ |
10,027,052 |
$ |
15,379,552 |
||
|
Total liabilities |
$ |
2,618,514 |
$ |
2,618,514 |
||
|
Total stockholders’ equity |
$ |
7,408,538 |
$ |
12,761,038 |
||
____________
(1) The pro forma as adjusted column reflects the sale of 1,500,000 shares of our common stock offered in this offering, assuming an initial public offering price of $4.50 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
(2) A $1.00 increase or decrease in the assumed initial public offering price of $4.50 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase or decrease the pro forma as adjusted amount of each of cash, cash equivalents and investments, working capital, total assets and total stockholders’ equity by $1.4 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions. An increase or decrease of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase or decrease the pro forma as adjusted amount of each of cash, cash equivalents and investments, working capital, total assets and total stockholders’ equity by $4.2 million, assuming no change in the assumed initial public offering price per share and after deducting estimated underwriting discounts and commissions. This pro forma as adjusted information is illustrative only and will depend on the actual initial public offering price and other terms of this offering determined at pricing.
(3) We define working capital as current assets less current liabilities. See our consolidated financial statements for further details regarding our current assets and current liabilities. See our financial statements and related notes included elsewhere in this prospectus for further details regarding our current assets and current liabilities.
8
An investment in our common stock involves a high degree of risk. In deciding whether to invest, you should carefully consider the following risk factors, as well as the financial and other information contained in this prospectus, including our consolidated financial statements and related notes. Any of the following risks could have a material adverse effect on our business, financial condition, results of operations or prospects and cause the value of our stock to decline, which could cause you to lose all or part of your investment. Additional risks and uncertainties of which we are unaware, or that we currently deem immaterial also may become important factors that affect us.
Risks Related to Our Limited Operating History, Financial Position and Need For Additional Capital
We have a very limited operating history, and we have incurred losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. We may never generate any revenue or become profitable or, if we achieve profitability, we may not be able to sustain it.
We were incorporated in September 2021 and we have a very limited operating history upon which you can evaluate our business and prospects. Our operations to date have been primarily focused on acquiring the assets of Molybdos (after participating in and being declared the winner of a competitive auction process under Section 45 of the South Africa Consumer Protection Act, 2008 for ZAR 11,000,000, which at the then current exchange rate was approximately USD 734,000)) and in-licensing intellectual property rights related to the production of Molybdenum-100 (a non-radioactive isotope we believe may have applications primarily in the medical industry) and Uranium-235 (an isotope of uranium we believe may have application in the clean, efficient and carbon-free energy industry) using the ASP technology, organizing and staffing our company, research and development activities, business planning, raising capital, and providing general and administrative support for these operations. In July 2022, we acquired assets comprising a dormant Silicon-28 aerodynamic separation processing plant from Klydon for ZAR 6,000,000 (which at the then current exchange rate was approximately USD 364,000), which will be payable to Klydon on the later of 180 days of the acquisition and the date on which the assets generate any revenues of any nature. We have not yet built a functioning Mo-100 or U-235 manufacturing plant or even demonstrated the ability to produce Mo-100 or U-235 using the ASP technology. We have not yet demonstrated an ability to overcome many of the risks and uncertainties frequently encountered by companies in the medical, technology and energy industries, including an ability to obtain applicable regulatory approvals, manufacture any isotopes at commercial scale (or arrange for a third party to do so on our behalf), or conduct sales and marketing activities necessary for successful isotope commercialization. In addition, we have not yet sought any regulatory approval that may be necessary for application of Mo-100 that we may develop using the ASP process in the medical industry. Consequently, any predictions about our future performance may not be as accurate as they would be if we had a history of successfully developing and commercializing isotopes.
Investment in isotope enrichment technology is highly speculative because it entails substantial upfront capital expenditures and significant risk that any potential isotopes will fail to demonstrate adequate utility or effectiveness in the targeted application (or for medical indications, an acceptable safety profile), gain regulatory approval, if applicable, and become commercially viable. We have no products approved for commercial sale and have not generated any revenue to date, and we continue to incur significant research and development and other expenses related to our ongoing operations. As a result, we are not profitable and have incurred losses since our inception in September 2021. For the period from September 13, 2021 (inception) through December 31, 2021, we reported a net loss of $2.6 million. For the six-month period ended June 30, 2022, we reported a net loss of $1.7 million. As of June 30, 2022, we had an accumulated deficit of $4.3 million.
We expect to continue to incur significant losses for the foreseeable future, and we expect these losses to increase as we:
• continue to invest in our research and development activities;
• seek applicable regulatory approvals for any future isotopes that we may successfully develop;
• experience any delays or encounter any issues with any of the above, including but not limited to failed research and development activities, safety issues or other regulatory challenges, the risk of which in each case may be exacerbated by the ongoing COVID-19 pandemic;
9
• hire additional engineering and production personnel and build our internal resources, including those related to audit, patent, other legal, regulatory and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and investor and public relations costs;
• obtain, expand, maintain, enforce and protect our intellectual property portfolio;
• establish a sales, marketing and distribution infrastructure and establish manufacturing capabilities, whether alone or with third parties, to commercialize future isotopes (assuming receipt of applicable regulatory approvals), if any; and
• operate as a public company.
We expect limited commercial activity for Mo-100 in the United States during the next two to three years and we anticipate that most of our initial revenues from future sales of our Mo-100 will be derived from countries in Asia and EMEA (Europe, Middle East and Africa). To become and remain profitable, we must succeed in developing and eventually commercializing isotopes that generate significant revenue. This will require us to be successful in a range of challenging activities, including completing research and development activities relating to our ASP technology, obtaining applicable regulatory approval for future isotopes, if any, and manufacturing, marketing and selling any future isotopes (assuming receipt of applicable regulatory approvals). We are only in the preliminary stages of most of these activities. We may never succeed in these activities and, even if we do, may never generate revenues that are significant enough to achieve profitability. Because of the numerous risks and uncertainties associated with chemical isotopes separation, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our future isotopes or even continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
Our future prospects are tied directly to the diagnostic medical imaging industry and depend on our ability to successfully introduce our Mo-100 and adapt to a changing technology and medical practice landscape.
The field of diagnostic medical imaging is dynamic, with new products, including equipment, software and products, continually being developed and existing products continually being refined. New hardware (scanners), software or agents in a given diagnostic modality may be developed that provide benefits superior to the then-dominant hardware, software and agents in that modality, resulting in commercial displacement of the existing radiotracers. For example, alternate scanners and radiotracers could be introduced. Similarly, changing perceptions about comparative efficacy and safety, as well as changing availability of supply may favor one agent over another or one modality over another. In addition, new or revised appropriate use criteria developed by professional societies, to assist physicians and other health care providers in making appropriate imaging decisions for specific clinical conditions, can and have reduced the frequency of and demand for certain imaging modalities and imaging agents. Technological obsolescence in any of the medical imaging products that would use the Mo-100 that we plan to manufacture could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We may not realize the anticipated benefits of previous acquisitions.
The success of the company will depend in large part on the success of our management in integrating the acquired assets into the company. In October 2021, our subsidiary in South Africa acquired the assets of Molybdos after participating in and being declared the winner of a competitive auction process under Section 45 of the South Africa Consumer Protection Act, 2008 for ZAR 11,000,000 (which at the then current exchange rate was approximately USD 734,000), plus value added tax (VAT) levied by the government of South Africa at the rate of 15% and auctioneers’ commission at the rate of 10%. We have not yet built a functioning Mo-100 or U-235 manufacturing plant or even demonstrated the ability to produce Mo-100 or U-235 using the assets acquired at the business rescue auction. We will not know whether the assets that we acquired will work according to our expectations until we have completed construction of the Molybdos plant as contemplated by our turnkey contract with Klydon (see the section of this prospectus entitled “Certain Relationships and Related Party Transactions — Our Relationship with Klydon Proprietary Limited — Turnkey Contract”). In July 2022, we acquired assets comprising a dormant Silicon-28 aerodynamic separation processing plant from Klydon
10
located in Pretoria, South Africa for ZAR 6,000,000 (which at the then current exchange rate was approximately USD 364,000). We intend to explore commercial opportunities for Silicon-28 and other light isotopes that may be produced using these assets. Our failure to achieve the integration of the acquired assets into the company and to commercialize the assets could result in our failure to realize the anticipated benefits of those acquisitions and could impair our results of operations, profitability and financial results.
We currently have no customers or sales, but we expect to be heavily dependent on a few large customers to generate a majority of our revenues for our Mo-100. Our operating results could be adversely affected by a reduction in business with our future significant customers.
We currently have no customers or sales. However, we expect to rely on a limited number of customers outside of the United States to purchase any Mo-100 that we develop using the ASP technology under long-term contracts. Our future key customers may stop ordering our Mo-100 at any time or may become bankrupt or otherwise unable to pay. The loss of any of our future key customers could result in lower revenues than we anticipate and could harm our business, financial condition or results of operations.
Our independent registered public accounting firm’s report contains an explanatory paragraph that expresses substantial doubt about our ability to continue as a “going concern.”
We incurred a net loss of $2.6 million for the period from September 13, 2021 (inception) through December 31, 2021 and a net loss of $1.7 million for the six-month period ended June 30, 2022. As of June 30, 2022, we had approximately $2.8 million in cash. We have yet to generate any revenues and we anticipate that our losses will continue for the foreseeable future. We cannot assure you that our plans to commercialize isotopes that we may develop will be successful. These factors, among others, raise substantial doubt about our ability to continue as a going concern. The financial statements contained elsewhere in this report do not include any adjustments that might result from our inability to continue as a going concern. Unless we can begin to generate material revenue, we may not be able to remain in business. We cannot assure you that we will raise enough money or generate sufficient sales to meet our future working capital needs.
Even if this offering is successful, we will require substantial additional capital to finance our operations, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate certain of our product development efforts or other operations.
We expect our expenses to increase substantially in connection with our ongoing and planned activities, particularly as we continue our research and development activities, seek applicable regulatory approvals for any future isotopes that we may successfully develop, and expand our organization by hiring additional personnel. In addition, following the closing of this offering, we expect to incur additional costs associated with operating as a public company.
As of June 30, 2022, our cash was approximately $2.8 million. We believe, based on our current operating plan, that the net proceeds from this offering, together with our existing cash and cash equivalents, will be sufficient to fund our operations for at least the next 12 months after the closing of the offering. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, third-party funding, marketing and distribution arrangements, as well as other collaborations, strategic alliances and licensing arrangements, or any combination of these approaches.
In any event, we will require substantial additional capital to support our business operations as we pursue additional research and development activities related to our ASP technology and seek applicable regulatory approval of our any future isotopes, and otherwise to support our continuing operations. In addition, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution (assuming receipt of applicable regulatory approvals for our future isotopes). Even if we believe we have sufficient capital for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations. Any additional capital raising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our future isotopes (assuming receipt of applicable regulatory approvals).
11
Additional funding may not be available on acceptable terms, or at all. We have agreed to pay the underwriter of this offering “tail compensation” equal to 8.0% of the aggregate gross proceeds received by us from the sale of our common stock in any private or public offering or other financing or capital-raising transaction of any kind within the 12 month period following the effective date of the registration statement of which this prospectus is a part. As a result of the COVID-19 pandemic and actions taken to slow its spread, the global credit and financial markets have experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability. If the equity and credit markets deteriorate, it may make any necessary debt or equity financing more difficult, more costly or more dilutive. If we do not raise additional capital in sufficient amounts, we may be prevented from pursuing development and commercialization efforts, which will harm our business, operating results and prospects.
Raising additional capital or acquiring or licensing assets by issuing equity or debt securities may cause dilution to our stockholders, and raising funds through lending and licensing arrangements may restrict our operations or require us to relinquish proprietary rights.
We may seek additional capital through a combination of public and private equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. The incurrence of indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional capital through future collaborations, strategic alliances or third-party licensing arrangements, we may have to relinquish valuable rights to our intellectual property, future revenue streams, research programs or future isotopes, or grant licenses on terms that may not be favorable to us.
If we are unable to raise additional capital when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts, or grant rights to develop and market our future isotopes (assuming receipt of applicable regulatory approvals for our future isotopes) that we would otherwise develop and market ourselves.
Risks Related to the Development and Commercialization of Our Future Isotopes
We are early in our research and development efforts for Mo-100 and U-235 using the ASP technology. If we are unable to advance our future isotopes in development, obtain applicable regulatory approval and ultimately commercialize our future isotopes, or experience significant delays in doing so, our business will be materially harmed.
We are early in our research and development efforts and currently have only Mo-100, a non-radioactive isotope we believe may have applications primarily in the medical industry (for use by radiopharmacies, hospitals, clinics and others in the medical community to prepare various nuclear imaging agents), in development using the ASP technology. We initiated the initial proof of concept stage (Phase 1) of the Mo-100 development plan in October 2021 and while we expect to complete the proof of concept stage during the second half of 2022, it is possible that the proof of concept stage will take longer than anticipated to complete due to unexpected delays.
We also plan to begin enrichment of uranium, which is a chemical element we believe may have application in the clean, efficient and carbon-free energy industry, using the ASP technology. We are in the planning stage of research and development activities for enriched uranium. If we are unable to advance our future isotopes in development, obtain applicable regulatory approval and ultimately commercialize our future isotopes (assuming receipt of applicable regulatory approvals), or experience significant delays in doing so, our business will be materially harmed.
Our ability to generate product revenues will depend heavily on the success of our research and development activities, receipt of applicable regulatory approvals, and eventual commercialization of our future isotopes (assuming receipt of applicable regulatory approvals and compliance with all applicable regulatory authorities).
The success of our business, including our ability to finance our company and generate any revenue in the future, will primarily depend on the successful development, regulatory approval and commercialization of our currently planned future isotopes, Mo-100 and enriched uranium, which may never occur.
12
We will have to be successful in a range of challenging activities, including completing research and development activities relating to our ASP technology, obtaining applicable regulatory approval for future isotopes, if any, and manufacturing, marketing and selling any future isotopes (assuming receipt of applicable regulatory approvals). We are only in the preliminary stages of most of these activities. If we are unable to succeed in these activities, we may not be able to generate sufficient revenue to continue our business.
We rely on a limited number of suppliers to provide us components and a material interruption in supply could prevent or limit our ability to execute our strategic plan and development programs in the expected timeframe.
We depend upon a limited number of third-party suppliers located for certain components required to construct the centrifuges and other equipment for the enrichment plant that is being constructed in South Africa. To date, we have been able to obtain the required components for our centrifuges without any significant delays or interruptions, except for certain delays related to COVID-19. If we lose any of these suppliers, we may be required to find and enter into supply arrangements with one or more replacement suppliers. Obtaining alternative sources of supply could involve significant delays and other costs and these supply sources may not be available to us on reasonable terms or at all. Any disruption of supplies could delay completion of the enrichment plant in South Africa, which could adversely affect our ability to execute our strategic plan and development programs in the expected timeframe.
Our business, financial and operating performance could be adversely affected by epidemics and other health related issues including but not limited to the coronavirus disease 2019 (“COVID-19”) pandemic.
The global outbreak of COVID-19 has negatively affected global economies, disrupted supply chains, and has resulted in significant travel, transport, and other restrictions. The COVID-19 outbreak has disrupted the supply chains and day-to-day our operations (and the operations of our suppliers and contractors (including Klydon), which could materially adversely affect our operations). In this regard, global supply chains and the timely availability of components imported to South Africa from the United States, countries in Europe or other nations could be materially disrupted by quarantines, slowdowns or shutdowns, border closings, and travel restrictions resulting from the global COVID-19 pandemic or other global pandemic or health crises. Further, impacts of COVID-19 infections and other COVID-19 pandemic related impacts on our management and workforce, or our suppliers and contractors (including Klydon), could adversely impact our business. While we have taken steps to protect our workforce and carry on operations, we may not be able to mitigate all of the potential impacts. We anticipate increased costs related to, or resulting from, the COVID-19 pandemic, due to, among other things, delays in supplier deliveries, impacts of travel restrictions, site access and quarantine requirements.
In the event that the COVID-19 pandemic prevents our employees or our contractors from working in-person at our facility in South Africa or our suppliers are unable to provide goods and services on the schedule we anticipated, the impacts on our schedule and costs could be material. The ultimate impact of the COVID-19 pandemic on our operations, including our ability to execute our strategic plan and development programs in the expected timeframe, remains uncertain and will depend on future pandemic-related developments, including the duration of the pandemic and any potential subsequent variants of COVID-19 and related government actions to prevent and manage disease spread, all of which are uncertain and cannot be predicted. The long-term impacts of the COVID-19 pandemic on us, our contractors and suppliers that could impact our business are also difficult to predict but could adversely affect our business, results of operations, and prospects.
Development activities at our facility in South Africa could be disrupted for a variety of reasons, which could prevent us from completing our development activities.
A disruption in development activities at our facility in South Africa could have a material adverse effect on our business. Disruptions could occur for many reasons, including power outages, fire, natural disasters, weather, unplanned maintenance or other manufacturing problems, public health crises (including, but not limited to, the COVID-19 pandemic), disease, strikes or other labor unrest, transportation interruption, government regulation, political unrest or terrorism. Alternative facilities with sufficient capacity or capabilities may not be available, may cost substantially more or may take a significant time to start production, each of which could negatively affect our business and financial performance.
13
Risks associated with the in-licensing of the ASP technology for development of Mo-100 or enriched uranium could cause substantial delays in the development of our future isotopes.
Prior to October 2021, as a company we had no involvement with or control over the research and development of the ASP technology. We have relied and continue to rely on Klydon to conduct such research and development in accordance with the applicable legal, regulatory and scientific standards prior to the in-licensing of the ASP technology for development of Mo-100 or U-235. If the research and development processes or the results of the development programs prior to the in-licensing of the ASP technology for development of Mo-100 or U-235 prove to be unreliable, this could result in increased costs and delays in the development of our future isotopes, which could adversely affect any future revenue from these future isotopes (assuming receipt of applicable regulatory approvals).
Regulatory approval for production and distribution of radiopharmaceuticals used for medical imaging and therapeutic treatments may involve a lengthy and expensive process with an uncertain outcome.
Currently, the sale or use of Mo-100 is not regulated by a healthcare regulator, such as the FDA, European Medicines Agency (EMA) or comparable foreign regulatory authorities. However, products such as Mo-99 and Tc-99m that are produced from Mo-100 in a cyclotron or a linear accelerator are regulated by healthcare regulators. We expect radiopharmacies, hospitals, clinics and others in the medical community to produce the widely used medical radioisotope technetium-99m (Tc-99m) from the Mo-100 that we may produce using our ASP technology. Tc-99m is a diagnostic agent that is used by health care professionals with FDA-approved imaging devices to detect potential diseases like coronary artery disease and cancer, as well as evaluating lung, liver, kidney and brain function. When used with the appropriate diagnostic scanner device, such as a SPECT imaging system, the Tc-99m emits signals that are captured and produces an image of internal organs to detect various medical problems and contribute to diagnosis and treatment decisions. Our future customers who may use Mo-100 to produce radiopharmaceuticals will likely require regulatory approval for their products. To date, only one healthcare regulator (Canada) has approved the use of Tc-99m produced from Mo-100 via a cyclotron. Obtaining regulatory approval is expensive and can take many years to complete, and its outcome is inherently uncertain. Our customers’ regulatory approval process may not be conducted as planned or completed on schedule, if at all, and failure can occur at any time during the process.
In the future, we may need to obtain approval from the FDA, EMA or comparable foreign regulatory authorities prior to the sale of Mo-100 that we may produce using our ASP technology for use in medical imaging and therapeutic treatments. If we require FDA, EMA or other comparable foreign regulatory authorities to approve the sale of Mo-100 that we may produce using our ASP technology for medical imaging and therapeutic treatments, we must demonstrate the safety and utility or efficacy of our Mo-100. Obtaining regulatory approval is expensive and can take many years to complete, and its outcome is inherently uncertain. Our regulatory approval process may not be conducted as planned or completed on schedule, if at all, and failure can occur at any time during the process.
Our success depends on our future customers’ ability to successfully commercialize products that are produced from our isotopes.
Our customers operate in a competitive environment. If our customers are unable to successfully commercialize products that they produce from our isotopes, our business will be negatively impacted. Our customers may fail for a number of reasons including but not limited to pricing pressure from competing products and failure to gain regulatory approval for the production of their products from healthcare regulators.
Our success depends on our ability to adapt to a rapidly changing competitive environment in the nuclear industry.
The nuclear industry in general, and the nuclear fuel industry in particular, is in a period of significant change, which could significantly transform the competitive landscape we face. The uranium and isotope enrichment sector is competitive. Changes in the competitive landscape may adversely affect pricing trends, change customer spending patterns, or create uncertainty. To address these changes, we may seek to adjust our cost structure and efficiency of operations and evaluate opportunities to grow our business organically or through acquisitions and other strategic transactions. We are actively considering, and expect to consider from time to time in the future, potential strategic transactions, which could involve, without limitation, changes in our capital structure, acquisitions and/or dispositions of businesses or assets, joint ventures or investments in businesses, products or technologies.
14
In connection with any such transaction, we may seek additional debt or equity financing, contribute or dispose of assets, assume additional indebtedness, or partner with other parties to consummate a transaction. Any such transaction may not result in the intended benefits and could involve significant commitments of our financial and other resources. Legal and consulting costs incurred in connection with debt or equity financing transactions in development are deferred and subject to immediate expensing if such a transaction becomes less likely to occur. If the actions we take in response to industry changes are not successful, our business, results of operations and financial condition may be adversely affected.
We may explore strategic collaborations that may never materialize or may fail.
We intend to accelerate the development of our enriched uranium program by selectively collaborating with energy companies in the United States. We intend to retain significant technology, economic and commercial rights to our programs in key geographic areas that are core to our long-term strategy. As a result, we intend to periodically explore a variety of possible additional strategic collaborations in an effort to gain access to additional resources. At the current time, we cannot predict what form such a strategic collaboration might take. We are likely to face significant competition in seeking appropriate strategic collaborators, and strategic collaborations can be complicated and time consuming to negotiate and document. We may not be able to negotiate strategic collaborations on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional strategic collaborations because of the numerous risks and uncertainties associated with establishing them.
If the market opportunities for our future isotopes are smaller than we estimate (even assuming receipt of any required regulatory approval), our business may suffer.
We are currently focused on producing Mo-100 using our ASP technology to meet critical patient healthcare needs and advance clinical research. We also plan to produce enriched uranium to meet the future needs of developers of U.S. advanced reactor technologies requiring HALEU. Our projections of the potential markets are based on estimates that have been derived from a variety of sources, including scientific literature and market research, and which may prove to be incorrect. We must be able to successfully acquire a significant market share in our potential markets to achieve profitability and growth. Customers may become difficult to gain access to, which would adversely affect our results of operations and our business.
We face substantial competition, which may result in others discovering, developing or commercializing isotopes before or more successfully than us.
The development and commercialization of radioisotopes and chemical elements is highly competitive. We face competition with respect to Mo-100 that we may produce using our ASP technology from established biotechnology and nuclear medicine technology companies and will face competition with respect to enriched uranium that we may seek to develop or commercialize in the future from innovative technology and energy companies. There are a number of large biotechnology and nuclear medicine technology companies that currently market and sell radioisotopes to radiopharmacies, hospitals, clinics and others in the medical community (Mo-99 is the active ingredient for Tc-99m-based radiopharmaceuticals used in nuclear medicine procedures). There are also a number of technology and energy companies that are currently seeking to develop HALEU. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
More established companies may have a competitive advantage over us due to their greater size, resources and institutional experience. In particular, these companies have greater experience and expertise in securing reimbursement, government contracts, relationships with key opinion leaders, obtaining and maintaining regulatory approvals and distribution relationships to market products. These companies also have significantly greater research and marketing capabilities than we do. If we are not able to compete effectively against existing and potential competitors, our business and financial condition may be harmed.
As a result of these factors, our competitors may complete development of isotopes before we are able to, which may limit our ability to develop or commercialize our future isotopes. Our competitors may also develop radioisotopes or technologies that are safer, more effective, more widely accepted and cheaper than ours, and may
15
also be more successful than us in manufacturing and marketing their isotopes. These appreciable advantages could render our future isotopes obsolete or non-competitive before we can recover the expenses of their development and commercialization.
Mergers and acquisitions in the technology and energy industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific, management and commercial personnel, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if the products that we or our customers may produce using the ASP technology receives regulatory approval, it may fail to achieve market acceptance by radiopharmacies, hospitals, clinics or others in the medical community necessary for commercial success.
Even if the Mo-100 that we may produce using the ASP technology, or Tc-99m or Mo-99 that we expect our future customers to produce using the Mo-100 that we plan to offer, receives regulatory approval, the isotopes may fail to gain sufficient market acceptance by radiopharmacies, hospitals, clinics and others in the medical community. If it does not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of Mo-100 that we may produce using the ASP technology, or the Tc-99m or Mo-99 that our future customers may produce, will depend on a number of factors, including but not limited to:
• the potential advantages compared to alternative radioisotopes;
• the timing of market introduction of the product as well as competitive products;
• effectiveness of sales and marketing efforts;
• the strength of our relationships with radiopharmacies, hospitals, clinics and others in the medical community;
• the cost in relation to alternative radioisotopes;
• our ability to offer Mo-100 that we may produce using the ASP technology for sale at competitive prices;
• the convenience and ease of use compared to alternative radioisotopes;
• the willingness of radiopharmacies, hospitals, clinics and others in the medical community to try an innovative radioisotope; and
• the strength of marketing and distribution support;
Our efforts to educate radiopharmacies, hospitals, clinics and others in the medical community on the benefits of Mo-100 that we may produce using the ASP technology may require significant resources and may never be successful.
Because we expect sales of Mo-100 that we may produce using the ASP technology (assuming receipt of applicable regulatory approvals for commercial sale) to generate substantially all of our revenues for the foreseeable future, the failure of Mo-100 that we may produce using the ASP technology (assuming receipt of applicable regulatory approvals for commercial sale) to find market acceptance would harm our business and could require us to seek additional financing.
We currently have no marketing and sales organization and have no experience as a company in commercializing products, and we may have to invest significant resources to develop these capabilities. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our products, we may not be able to generate product revenue.
We have no internal sales, marketing or distribution capabilities, nor have we commercialized a product. If the Mo-100 that we may produce using our ASP technology gains market acceptance and our customers receive regulatory approval for the isotopes they produce, we must build a marketing and sales organization with technical
16
expertise and supporting distribution capabilities to commercialize such product in the markets that we target, which will be expensive and time consuming, or collaborate with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. We currently plan to independently commercialize the Mo-100 that we may produce using our ASP technology (assuming receipt of applicable regulatory approvals) in the United States by establishing a focused sales force and marketing infrastructure. We may opportunistically seek additional strategic collaborations to maximize the commercial opportunities for our medical isotopes business outside of the United States. We have no prior experience as a company in the marketing, sale and distribution of isotopes and there are significant risks involved in building and managing a sales organization, including our ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel and effectively manage a geographically dispersed sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of these products. We may not be able to enter into collaborations or hire consultants or external service providers to assist us in sales, marketing and distribution functions on acceptable financial terms, or at all. In addition, our product revenues and our profitability, if any, may be lower if we rely on third parties for these functions than if we were to market, sell and distribute any products that we develop ourselves. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we are not successful in commercializing our isotopes, either on our own or through arrangements with one or more third parties, we may not be able to generate any future product revenue and we would incur significant additional losses.
Obtaining regulatory approval for either the Mo-100 that we may produce using the ASP technology, or the Tc-99m or Mo-99 that our future customers may produce using the Mo-100 that we plan to offer, in one jurisdiction does not mean that we or they will be successful in obtaining regulatory approval of such future products in other jurisdictions.
Currently, the production and distribution of Mo-100 does not require any regulatory licenses from healthcare regulators. Healthcare regulators frequently change such requirements, and it is possible that in the future Mo-100 may be regulated as a healthcare product. Obtaining such regulatory licenses, if required, may be a timely and costly process and could materially impact our ability to commercialize the Mo-100 that we plan to offer. Obtaining regulatory approval of the Mo-100 that we may produce using the ASP technology in one jurisdiction does not guarantee that we will be able to obtain regulatory approval in any other jurisdiction. For example, even if the FDA grants regulatory approval of the Mo-100 that we may produce using the ASP technology, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion and reimbursement of such future product in those countries. However, a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States.
Obtaining foreign regulatory approvals and establishing and maintaining compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of the Mo-100 that we may produce using the ASP technology. Products such as Tc-99m and Mo-99 that may be produced by our future customers using the Mo-100 that we plan to offer will likely require regulatory licenses in most regions. Healthcare regulators frequently change such requirements, and it is unclear what each healthcare regulator will require. To date, only one region (Canada) has approved the use of Tc-99m that has been produced from Mo-100 in a cyclotron. Obtaining such regulatory licenses, if required, may be a timely and costly process and could materially impact the ability of our future customers to operate and use the Mo-100 that we plan to offer. Obtaining regulatory approval in one jurisdiction does not guarantee that we or they will be able to obtain regulatory approval in any other jurisdiction.
If we or any future collaborator fail to comply with the regulatory requirements in international markets or fail to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of the Mo-100 that we may produce using the ASP technology will be harmed.
17
Product liability lawsuits against us could cause us to incur substantial liabilities and could limit commercialization of any isotopes that we may develop.
We face an inherent risk of product liability exposure if we commercialize any isotopes that we may develop. If we cannot successfully defend ourselves against claims that any such isotopes caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
• decreased demand for any isotopes that we may develop;
• loss of revenue;
• substantial monetary awards to patients;
• significant time and costs to defend the related litigation;
• a diversion of management’s time and our resources;
• initiation of investigations by regulators;
• the inability to commercialize any isotopes that we may develop;
• injury to our reputation and significant negative media attention; and
• a decline in our share price.
Any product liability insurance coverage that we obtain and maintain may not be adequate to cover all liabilities that we may incur. We anticipate that we will need to increase our insurance coverage each time we commence a clinical trial and if we successfully commercialize any isotopes. Insurance coverage is increasingly expensive. We may not be able to obtain or maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Risks Related to Regulatory Compliance
Our business is and could become subject to a wide variety of extensive and evolving laws and regulations. Failure to comply with such laws and regulations and failure to obtain licenses, approvals and permits that may be required to execute on our strategy and develop our company’s business could have a material adverse effect on our business.
We are subject to a wide variety of laws and regulations relating to various aspects of our business, including with respect to the development of the ASP technology and our future isotopes, employment and labor, health care, tax, privacy and data security, health and safety, and environmental issues. Laws and regulations at the South African and foreign, federal, state and local levels frequently change, especially in relation to new and emerging industries, and we cannot always reasonably predict the impact of, or the ultimate cost of compliance with, current or future regulatory or administrative changes. In South Africa, our Mo-100 enrichment facility is heavily regulated. South Africa is a signatory to the International Atomic Energy Agency (“IAEA”) conventions and has adopted safety standards from the IAEA. The design, construction and operation of the Mo-100 enrichment plant are highly regulated and require government licenses, approvals and permits, and may be subject to the imposition of conditions. In some cases, these licenses, approvals and permits entail periodic review and inspections. While we and Klydon have received all licenses, approvals and permits required to build and operate our Mo-100 enrichment facility in South Africa, we cannot predict whether the conditions associated with such licenses, approvals and permits will be maintained. For example, each of Klydon and ASP Isotopes South Africa (Proprietary) Limited has received from the South African Council for The Non-Proliferation of Weapons of Mass Destruction (1) a registration certificate (which are valid for two years from the date of issuance) and (2) a Manufacturing and Services Permit. The permits provide that the Non-Proliferation Secretariat will conduct at least two industry visits in June and November (or as arranged) of every year. Each of the permits includes numerous conditions, including, for example, the obligation to keep the Council updated or informed on all separation projects at all times and at least through biannual declarations, which must be done through correspondence to the Council at the end of April and September every year. The permit issued to ASP Isotopes South Africa (Proprietary) Limited
18
includes additional specific information requirements related to (i) the progress on the design and construction of the Mo-100 separation plant, (ii) the progress on the manufacturing of Molybdenum separation elements, and (iii) the commissioning of the plant. Each of the permits further provides that (i) any potential export of controlled goods and technology should be requested at an early stage through a Provisional Export Guidance Request, (ii) all isotope separation applications remain controlled regardless of the isotope atomic mass and will be dealt with on a case-by-case basis, and (iii) any ultimate transfer of these controlled goods and technology will be subject to the issuance of a permit by the Council as required in terms of the Non-Proliferation Act and related Government Notices and Regulations.
In addition, we cannot assure you that we will be able to obtain, on a timely basis or at all, any additional licenses, approvals and permits that may be required to execute on our strategy and develop our company’s business, including any such licenses, approvals and permits that may be required to introduce Mo-100 produced using ASP technology into the market and to begin the enrichment of uranium to demonstrate our capability to produce HALEU using the ASP technology.
Changes in law or the imposition of new or additional regulations or permit requirements that impact our business could negatively impact our performance in various ways, including by limiting our ability to collaborate with partners or customers or by increasing our costs and the time necessary to obtain required authorization. We monitor new developments and devote a significant amount of management’s time and external resources to compliance with these laws and regulations. We cannot assure you, however, that we are and will remain in compliance with all such requirements and, even when we believe we are in compliance, a regulatory agency may determine that we are not. In addition, we cannot assure you that we will be obtain all licenses, approvals and permits that may be required to execute on our strategy and develop our company’s business as currently contemplated. Failure by us, our employees, affiliates, partners or others with whom we work to comply with applicable laws and regulations or to obtain or comply with necessary licenses, approvals and permits could result in administrative, civil, commercial or criminal liabilities, including suspension or debarment from government contracts or suspension of our export/import privileges. Failure by us, our employees, affiliates, partners or others with whom we work to comply with the permits issued to us by the South African Council for The Non-Proliferation of Weapons of Mass Destruction could result in disruption of our development activities at our facility in South Africa, which could prevent us from completing our development activities.
If technology developed for the purposes of enriching isotopes can be applied to the creation or development of weapons-grade materials, then our technology may be considered “dual use” technology and be subject to limitations on public disclosure or export.
Our research and development of isotope enrichment is dedicated not only to producing Mo-100 for use in nuclear medical diagnostic procedures and concentrating uranium in the isotope uranium-235 for use in nuclear energy, but also to safeguarding any information with broad, dual-use potential that could be inappropriately applied. Enrichment is among the most sensitive nuclear technologies because it can produce weapon-grade materials. The ASP technology may be considered dual use and could be subject to export control, for example under the Wassenaar Arrangement.
Risks Related to Our Intellectual Property
Our intellectual property is not protected through patents or formal copyright registration. As a result, we do not have the full benefit of patent or copyright laws to prevent others from replicating the ASP technology.
Neither we nor Klydon have yet protected our respective intellectual property rights through patents or formal copyright registration, and neither we nor Klydon currently have any patent applications pending. To date, we and Klydon have relied exclusively on trade secrets and other intellectual property laws, non-disclosure agreements with our respective employees, consultants, vendors, potential customers and other relevant persons and other measures to protect our intellectual property, and intend to continue to rely on these and other means. As we intend to transition into the commercialization of Mo-100, we envision our intellectual property and its security becoming more vital to our future. Until we protect our intellectual property through patent, trademarks and registered copyrights, we may not be able to protect our intellectual property and trade secrets or prevent others from independently developing
19
substantially equivalent proprietary information and techniques or from otherwise gaining access to our intellectual property or trade secrets. In such an instance, our competitors could produce products that are nearly identical to ours resulting in us selling less products or generating less revenue from our sales.
We may be unable to adequately protect our intellectual property and proprietary rights and prevent others from making unauthorized use of our products and technology.
Our success and competitiveness depend, in significant part, on our ability to protect our intellectual property rights, including the ASP technology and certain other practices, tools, technologies and technical expertise we utilize in designing, developing, implementing and maintaining processes used in the development of our future isotopes. To date, we and Klydon have relied exclusively on trade secrets and other intellectual property laws, non-disclosure agreements with our respective employees, consultants, vendors, potential customers and other relevant persons and other measures to protect our intellectual property, and intend to continue to rely on these and other means.
For strategic reasons, neither we nor Klydon have yet protected our intellectual property by filing patent applications related to our technology, inventions and improvements. Even if we or Klydon filed patent applications and patents were granted, we cannot assure you we would be fully protected against third parties as those patents may not be sufficiently broad in their coverage, may not be economically significant, or may not provide us with any competitive advantage. Competitors may be able to design around any patents and develop isotope production techniques comparable or superior to the ASP technology. Furthermore, the filing of a patent would entail the disclosure of our know-how, and breaches of patent rights related to a wrongful use of this know-how would be difficult to enforce in the international landscape. We believe that our intellectual property strategy differs significantly from the strategies of others involved in the medical isotope industry, many of whom have extensive patent portfolios and rely heavily on intellectual property registrations to enforce their intellectual property rights. As a result of this discrepancy in strategy, we may be at a competitive disadvantage with respect to the strength of our intellectual property protection. Unlike others involved in the medical isotope industry, who generally have patents providing exclusive control over their innovations, we have no recourse against any entity that independently creates the same technology as ours or legitimately reverse-engineers our technology.
We generally enter into non-disclosure agreements with our employees, consultants and other parties with whom we have strategic relationships and business alliances. We cannot, however, assure you that these agreements will be effective in controlling access to and distribution of our technology and proprietary information. Since we do not protect our intellectual property by filing patent applications, we rely on our personnel to protect our trade secrets, know-how and other proprietary information to a greater degree than we would if we had patent protection for our intellectual property. In any jurisdiction in which our research and development is not protected by similar agreements, there is no protection against the manufacture and marketing of identical or comparable research and development by third parties, who are generally free to use, independently develop, and sell our developments and technologies without paying license or royalty fees. Furthermore, our former employees may perform work for our competitors and use our know-how in performing this work. In the event we scale our business by hiring additional personnel and entering into contracts with third parties, the risks associated with breaches of non-disclosure agreements, confidentiality agreements and other agreements pertaining to our technology and proprietary information will increase, and such breaches could have an adverse effect on our business and competitive position.
We may come to believe that third parties are infringing on, or otherwise violating, our intellectual property or other proprietary rights. To prevent infringement or unauthorized use, we may need to file infringement and/or misappropriation suits, which are expensive and time-consuming, could result in meritorious counterclaims against us and would distract management’s attention. In addition, in an infringement or misappropriation proceeding, a court may decide that one or more of our intellectual property rights is invalid, unenforceable, or both, in which case third parties may be able to use our technology without paying license fees or royalties. If we are unable to protect our intellectual property and proprietary rights, we may be unable to prevent competitors from using our own inventions and intellectual property to compete against us, and our business may be harmed.
20
We depend on intellectual property licensed from Klydon, the termination of which could result in the loss of significant rights, which would harm our business.
We are dependent on technology, know-how, and proprietary materials licensed from Klydon. We have an exclusive license from Klydon to use, develop, modify, improve, subcontract and sublicense certain intellectual property rights relating to the ASP technology for the production, distribution, marketing and sale of all isotopes produced using the ASP technology (the “Klydon license agreement”). The Klydon license agreement is royalty-free, has a term of 999 years and the license is worldwide for the development of the ASP technology and the distribution, marketing and sale of isotopes. Future production of isotopes is limited to member countries of the Nuclear Suppliers Group. Klydon has the right to terminate the exclusivity of the Klydon license agreement in the event that the licensee ceases to carry on activities related to isotope enrichment for a period longer than 24 consecutive months. Any termination of exclusivity under the Klydon license agreement will result in the loss of significant rights and will restrict our ability to develop and commercialize our planned isotopes. See the section of this prospectus entitled “Certain Relationships and Related Party Transactions — Our Relationship with Klydon Proprietary Limited — Omnibus Klydon License” for a description of the Klydon license agreement, which includes a description of the exclusivity termination provision. If we or Klydon fails to adequately protect this intellectual property, our ability to commercialize the isotopes, such as Mo-100 or uranium, that we may produce using ASP technology could suffer.
In addition, agreements under which we license intellectual property or technology to or from third parties may be complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected future isotopes. Our business also would suffer if our licensor fails to abide by the terms of the license, or if we are unable to enter into necessary licenses on acceptable terms. Moreover, our licensor may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise violating the licensor’s rights.
Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in our industry. Disputes may also arise between us and our licensors regarding intellectual property subject to a license agreement, including those relating to:
• the scope of rights granted under the license agreement and other interpretation-related issues;
• whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the license agreement;
• our right to sublicense rights to third parties under collaborative development relationships;
• whether we are complying with our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our future isotopes, and what activities satisfy those diligence obligations;
• the priority of invention of patented technology;
• the amount and timing of payments owed under license agreements; and
• the allocation of ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensor and by us and our partners.
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected future isotopes. We are generally also subject to all of the same risks with respect to protection of intellectual property that we license as we are for intellectual property that we own, which are described below. If we or our licensor fail to adequately protect this intellectual property, our ability to commercialize our future isotopes could suffer.
21
We have received a letter asserting that the license for the ASP technology granted to us from Klydon, which is critical to our business, may be invalid because these rights were already granted to a third party, Radfarma.
On October 25, 2022, we received a letter (the “NMS Letter”) from a law firm acting on behalf of Norsk Medisinsk Syklotronsenter AS (“NMS”), asserting, among other things, that the grant of a license to the ASP technology to us by Klydon violates a pre-existing exclusive sub-license to the ASP technology granted to Radfarma. The NMS Letter makes reference to: (1) a license agreement entered into on October 25, 2013 by Klydon and API Labs Pharmaceuticals (Proprietary) Limited (“API Labs”) to license the ASP technology for enriching certain isotopes of the element Molybdenum (“2013 API Labs License”); and (2) an exclusive sub-license to the ASP technology granted on October 1, 2019 to Radfarma, as licensee, by API Labs and SaPhotonica Limited (“SaPhotonica”), as licensors (the “2019 Radfarma Sub-License”). The NMS Letter states that Radfarma is a joint venture that is 45% owned by NMS and 45% owned by SaPhotonica. The NMS Letter also states that Klydon, SaPhotonica and ASP Isotopes Inc. are under common control by Dr. Hendrik Strydom and Einar Ronander.
The NMS Letter asserts, among other things, that the grant of a license to the ASP technology to us by Klydon (pursuant to license agreements entered into subsequent to the Radfarma Sub-License) violates a covenant in the 2019 Radfarma Sub-License that the licensors shall not be entitled, directly or indirectly, to use, grant or otherwise give the rights, or any similar rights, which were granted to Radfarma under the 2019 Radfarma Sub-License to any other person for use in the territory. “Territory” is defined in the 2019 Radfarma Sub-License as “the Kingdom of Norway for the construction of the 20-kilogram capacity plants; and means the international market where distribution agreements can be produced.” The NMS Letter asserts that while Klydon purported to give to us a license to market the ASP technology globally, these rights were already granted to Radfarma.
The NMS Letter includes a request for us to enter into discussions and an agreement with NMS based on terms proposed in previous correspondence from NMS which outlined a future collaboration on technology and product development. The NMS Letter does not include a threat of litigation against us or any parties to the 2013 API Labs License or 2019 Radfarma Sub-License. However, if the licensed rights granted to us are found to be invalid or unenforceable (in whole or in part), or if our exclusive license agreement with Klydon is terminated or Klydon, as licensor, fails to abide by the terms of our exclusive license agreement, our ability to commercialize our future isotopes would suffer and our business, results of operations and financial condition may be adversely affected.
Our license for the ASP technology with Klydon may be found to infringe third party intellectual property rights.
Third parties may in the future assert claims or initiate litigation related to their intellectual property rights in technology that is important to us, including the ASP technology. For example, on October 25, 2022, we received a letter (the “NMS Letter”) from a law firm acting on behalf of Norsk medisinsk syklotronsenter AS (“NMS”), asserting, among other things, that the grant of a license to the ASP technology to us by Klydon violates a pre-existing exclusive sub-license to the ASP technology granted to Radfarma, as more fully described in the risk factor above. The asserted claims, arbitration and/or litigation could include claims against us, our licensor (Klydon), or Klydon’s present or former sub-licensors alleging infringement of intellectual property rights with respect to the ASP technology on which our company relies. Regardless of the merit of the claims, they could be time consuming, resulting in costly arbitration or litigation and diversion of technical and management personnel, or require us to develop non-infringing technology or enter into license agreements. We cannot assure you that licenses will be available on acceptable terms, if at all. Furthermore, because of the potential for significant damage awards, which are not necessarily predictable, it is not unusual to find even arguably unmeritorious claims resulting in large settlements. If any infringement or other intellectual property claim made against us by any third party (including NMS or Radfarma) is successful, or if we fail to develop non-infringing technology or license the proprietary rights on commercially reasonable terms and conditions, our business, operating results and financial condition could be materially adversely affected.
If the ASP technology that we license from Klydon infringes the proprietary rights of other parties (including NMS or Radfarma), we could incur substantial costs, and we may have to take certain actions, including the following:
• obtain licenses, which may not be available on commercially reasonable terms, if at all;
• redesign our technology or processes to avoid infringement;
22
• stop using the subject matter claimed to be held by others;
• pay damages; or
• defend arbitration, litigation or administrative proceedings which may be costly whether we win or lose (and may be prohibitively expensive, particularly for a company of our size), and which could result in a substantial diversion of our financial and management resources.
In addition, in an infringement proceeding, a court or tribunal may decide that our asserted intellectual property is not valid or is unenforceable. An adverse determination in any litigation, arbitration or defense proceedings could put our licensed intellectual property at risk of being invalidated or interpreted narrowly. If the licensed rights granted to us are found to be invalid or unenforceable (in whole or in part), or if our exclusive license agreement with Klydon is terminated or Klydon, as licensor, fails to abide by the terms of our exclusive license agreement, our ability to commercialize our future isotopes would suffer and our business, results of operations and financial condition may be adversely affected.
We may enter into collaboration agreements and strategic alliances, and we may not realize the anticipated benefits of such collaborations or alliances.
We may wish to form collaborations in the future with respect to our future isotopes, but may not be able to do so or to realize the potential benefits of such transactions, which may cause us to alter or delay our development and commercialization plans. Research and development collaborations are subject to numerous risks, which may include the following:
• collaborators have significant discretion in determining the efforts and resources that they will apply to a collaboration, and may not commit sufficient efforts and resources, or may misapply those efforts and resources;
• collaborators may not pursue development and commercialization of future isotopes or may elect not to continue or renew development or commercialization programs;
• collaborators may delay, provide insufficient resources to, or modify or stop development activities for future isotopes;
• collaborators could develop or acquire products outside of the collaboration that compete directly or indirectly with our future isotopes;
• collaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability;
• disputes may arise between us and a collaborator that cause the delay or termination of the research, development or commercialization of our future isotopes, or that result in costly litigation or arbitration that diverts management attention and resources;
• collaborations may be terminated and, if terminated, may result in a need for additional capital and personnel to pursue further development or commercialization of the applicable future isotopes; and
• collaborators may own or co-own intellectual property covering our products that results from our collaborating with them, and in such cases, we may not have the exclusive right to commercialize such intellectual property.
The development and potential commercialization of our future isotopes will require substantial additional capital to fund expenses. We may form or seek further strategic alliances, create joint ventures or collaborations, or enter into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to our future isotopes, including in territories outside
23
the United States or for certain indications. These transactions can entail numerous operational and financial risks, including exposure to unknown liabilities, disruption of our business and diversion of our management’s time and attention in order to manage a collaboration or develop acquired products or technologies, incurrence of substantial debt or dilutive issuances of equity securities to pay transaction consideration or costs, higher than expected collaboration, acquisition or integration costs, write-downs of assets or goodwill or impairment charges, increased amortization expenses, difficulty and cost in facilitating the collaboration or combining the operations and personnel of any acquired business, impairment of relationships with key suppliers, manufacturers or customers of any acquired business due to changes in management and ownership and the inability to retain key employees of any acquired business. As a result, if we enter into acquisition or in-license agreements or strategic partnerships, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture, or if there are materially adverse impacts on our or the counterparty’s operations resulting from COVID-19, which could delay our timelines or otherwise adversely affect our business. We also cannot be certain that, following a strategic transaction or license, we will achieve the revenue or specific net income that justifies such transaction or such other benefits that led us to enter into the arrangement.
In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. We may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our future isotopes because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view our future isotopes as having the requisite potential to demonstrate safety and efficacy. If and when we collaborate with a third-party for development and commercialization of a future isotope, we can expect to relinquish some or all of the control over the future success of that future isotope to the third-party. Our ability to reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of our technologies, future isotopes and market opportunities. The collaborator may also consider alternative isotopes or technologies for similar applications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our future isotope. We may also be restricted under any license agreements from entering into agreements on certain terms or at all with potential collaborators.
As a result of these risks, we may not be able to realize the benefit of our existing collaborations or any future collaborations or licensing agreements we may enter into. In addition, we may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of such future isotope, reduce or delay one or more of our other development programs, delay the potential commercialization or reduce the scope of any planned sales or marketing activities for such future isotope, or increase our expenditures and undertake development, manufacturing or commercialization activities at our own expense. If we elect to increase our expenditures to fund development, manufacturing or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our future isotopes or bring them to market and generate product revenue.
We may be dependent on intellectual property licensed or sublicensed to us from, or for which development was funded or otherwise assisted by, government agencies, for development of our technology and future isotopes. Failure to meet our own obligations to our licensor or upstream licensors, including such government agencies, may result in the loss of our rights to such intellectual property, which could harm our business.
Government agencies may provide funding, facilities, personnel or other assistance in connection with the development of the intellectual property rights owned by or licensed to us. Such government agencies may have retained rights in such intellectual property, including the right to grant or require us to grant mandatory licenses or sublicenses to such intellectual property to third parties under certain specified circumstances, including if it is necessary to meet health and safety needs that we are not reasonably satisfying or if it is necessary to meet requirements for public use specified by federal regulations, or to manufacture products in the United States. Any exercise of such rights, including with respect to any such required sublicense of these licenses could result in the loss of significant rights and could harm our ability to commercialize licensed products.
24
If we are unable to obtain patent protection for our future isotopes, or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets.
We anticipate that Klydon will file patent applications both in the United States and in other countries, as appropriate. However, we cannot predict:
• if and when any patents will be issued;
• the degree and scope of protection any issued patents will afford us against competitors, including whether third parties will find ways to invalidate or otherwise circumvent our patents;
• whether others will apply for or obtain patents claiming aspects similar to those covered by our patents and patent applications;
• whether we will need to initiate litigation or administrative proceedings to defend our patent rights, which may be costly whether we win or lose; or
• whether the patent applications that we own, or in-license will result in issued patents with claims that cover our future isotopes or uses thereof in the United States or in foreign countries.
We currently rely upon a combination of trade secret protection and confidentiality agreements to protect the intellectual property related to our isotope development techniques and future isotopes. Our success will depend in large part on our (or Klydon, as our licensor) ability to obtain and maintain patent protection in the United States and other countries with respect to the ASP technology. We expect Klydon to seek to protect its proprietary position by filing patent applications in the United States and abroad related to its current and future development programs and future isotopes to the extent permitted by applicable law. Our exclusive license agreement with Klydon provides that additional patents, knowhow and improvements in the ASP technology that may be developed in the future will be considered part of the intellectual property rights granted under the license. The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner, including as a result of the COVID-19 pandemic impacting our or our licensors’ operations.
It is possible that we (or Klydon, as our licensor) will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. The patent applications that we own or have in-license may fail to result in issued patents with claims that cover our future isotopes in the United States or in foreign countries. There is no assurance that all of the potentially relevant prior art relating to our (or Klydon’s) patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover the ASP technology, third parties may challenge their scope, validity, or enforceability, which may result in such patents being narrowed, invalidated, or held unenforceable. Any successful opposition to these patents or any other patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any future isotopes using the ASP technology. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a future isotope could be reduced.
If the patent applications we hold or have in-licensed with respect to our development programs fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity for the ASP technology, it could dissuade companies from collaborating with us, and threaten our ability to commercialize, isotopes produced using the ASP technology. Any such outcome could have a negative effect on our business.
Even if we obtain patents covering the ASP technology or our methods, we may still be barred from making, using and selling such technology or methods because of the patent rights of others. Others may have filed, and in the future may file, patent applications covering technology or methods that are similar or identical to ours, which could materially affect our ability to successfully develop our technology or to successfully commercialize any isotopes alone or with collaborators.
Patent applications in the United States and elsewhere are generally published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our platform technologies and methods could have been filed
25
by others without our knowledge. Additionally, pending claims in patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our platform technologies. These patent applications may have priority over patent applications filed by us.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other government fees on patents and/or applications will be due to be paid to the USPTO and various government patent agencies outside of the United States over the lifetime of our owned and licensed patents and/or applications and any patent rights we may own or license in the future. We will rely on our outside counsel, patent annuity service providers, or our licensing partners to pay these fees due to non-U.S. patent agencies. The USPTO and various non-U.S. government patent agencies require compliance with several procedural, documentary, and other similar provisions during the patent application process. We will employ reputable law firms and other professionals to help us comply and we will also be dependent on Klydon (as our licensor) to take the necessary action to comply with these requirements with respect to our licensed intellectual property. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the market and this circumstance could harm our business.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a negative impact on the success of our business.
Our commercial success depends, in part, upon our ability and the ability of our current or future collaborators to develop, manufacture, market and sell our future isotopes and use our proprietary technologies without infringing the proprietary rights and intellectual property of third parties. The technology industry is characterized by extensive and complex litigation regarding patents and other intellectual property rights. Our future isotopes and other proprietary technologies we may develop may infringe existing or future patents owned by third parties. We may in the future become party to, or be threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our future isotopes and technology, including interference proceedings, post grant review and inter partes review before the USPTO. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future, regardless of their merit. There is a risk that third parties may choose to engage in litigation with us to enforce or to otherwise assert their patent rights against us. Even if we believe such claims are without merit, a court of competent jurisdiction could hold that these third-party patents are valid, enforceable and infringed, which could have a negative impact on our ability to commercialize our future isotopes. In order to successfully challenge the validity of any such U.S. patent in federal court, we would need to overcome a presumption of validity. As this burden is a high one requiring us to present clear and convincing evidence as to the invalidity of any such U.S. patent claim, there is no assurance that a court of competent jurisdiction would invalidate the claims of any such U.S. patent. If we are found to infringe a third party’s valid and enforceable intellectual property rights, we could be required to obtain a license from such third party to continue developing, manufacturing and marketing our future isotope(s) and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. We could be forced, including by court order, to cease developing, manufacturing and commercializing the infringing technology or future isotope. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent or other intellectual property right. A finding of infringement could prevent us from manufacturing and commercializing our future isotopes or force us to cease some or all of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business, financial condition, results of operations and prospects.
Third parties asserting their patent or other intellectual property rights against us may seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our future isotopes or force us to cease some of our business operations. Defense of these claims, regardless of their
26
merit, would involve substantial litigation expense and would be a substantial diversion of management and other employee resources from our business, cause development delays, and may impact our reputation. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties, or redesign our infringing products, which may be impossible on a cost-effective basis or require substantial time and monetary expenditure. In that event, we would be unable to further develop and commercialize our future isotopes, which could harm our business significantly. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may be subject to claims asserting that our employees, consultants or advisors have wrongfully used or disclosed alleged trade secrets of their current or former employers or claims asserting ownership of what we regard as our own intellectual property.
Certain of our employees, consultants or advisors are currently, or were previously, employed at universities or other technology companies, including Klydon. Although we try to ensure that our employees, consultants and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these individuals or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property.
Reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
If we rely on third parties to manufacture or commercialize our future isotopes, or if we collaborate with additional third parties for the development of our future isotopes, we must, at times, share trade secrets with them. We may also conduct joint research and development programs that may require us to share trade secrets under the terms of our research and development partnerships or similar agreements. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, services agreements, consulting agreements or other similar agreements with our advisors, employees, third-party contractors and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, including our trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure could have an adverse effect on our business and results of operations.
In addition, these agreements typically restrict the ability of our advisors, employees, third-party contractors and consultants to publish data potentially relating to our trade secrets. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of our agreements with third parties, independent development or publication of information by any third-party collaborators. A competitor’s discovery of our trade secrets could harm our business.
Confidentiality agreements with employees and third parties may not prevent unauthorized disclosure of trade secrets and other proprietary information.
In addition to the protection afforded by patents, we seek to rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce, and any other elements of our future isotopes, technology and product
27
discovery and development processes that involve proprietary know-how, information, or technology that is not covered by patents. Any disclosure, either intentional or unintentional, by our employees, the employees of third parties with whom we share our facilities or third-party consultants and vendors that we engage, or misappropriation by third parties (such as through a cybersecurity breach) of our trade secrets or proprietary information could enable competitors to duplicate or surpass our technological achievements, thus eroding our competitive position in our market. Because we expect to rely on third parties in the development and manufacture of our future isotopes, we must, at times, share trade secrets with them. Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Trade secrets and confidential information, however, may be difficult to protect. We seek to protect our trade secrets, know-how and confidential information, including our proprietary processes, in part, by entering into confidentiality agreements with our employees, consultants, outside scientific advisors, contractors, and collaborators. With our consultants, contractors, and outside scientific collaborators, these agreements typically include invention assignment obligations. We cannot guarantee that we have entered into such agreements with each party that may have or has had access to our trade secrets or proprietary technology and processes. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, outside scientific advisors, contractors, and collaborators might intentionally or inadvertently disclose our trade secret information to competitors. In addition, competitors may otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third-party, we would have no right to prevent them from using that technology or information to compete with us. Furthermore, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent unauthorized material disclosure of our intellectual property to third parties, or misappropriation of our intellectual property by third parties, we may not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, operating results, and financial condition.
Risks Related to Our Dependence on Third Parties
Klydon currently performs or supports many of our operating activities and will continue to do so after the closing of this offering pursuant to a turnkey contract, and if we are unable to replicate or replace these functions if this services agreement is terminated, our operations could be adversely affected.
In November 2021, we entered into a turnkey contract with Klydon (Turnkey Contract). Under this agreement, Klydon has been appointed to supply to ASP Isotopes South Africa (Proprietary) Limited a complete turnkey Molybdenum-100 enrichment plant. The activities to be undertaken or performed by Klydon include: taking control of the assets acquired by us in the Molybdos Business Rescue Auction; the design of a Molybdenum-100 enrichment facility with target manufacturing capability of 20 Kg p.a of 95% and above enriched Molybdenum isotope; the supply of components, equipment and labor required for 20 Kg p.a.; the installation, testing and commissioning of the Molybdenum enrichment plant, including production of targets to be used by customers in cyclotrons; securing all required approvals, regulatory authorizations and other required consents for the operation of the plant; providing training to local ASP Isotopes South Africa (Proprietary) Limited personnel to enable them to operate the plant going forward; and providing warranties in relation to the performance targets of the plant which are required to be met. Klydon will be responsible for liaising with the relevant South African authorities including the South African Non Proliferation Council, the Nuclear Suppliers Group and International Atomic Energy Agency to ensure that the Turnkey Contract and the Molybdenum-100 enrichment plant are compliant with international laws and guidelines. Because our company does not yet have sufficient internal capabilities to perform these functions, we are substantially dependent on the Turnkey Contract for the operation of our company.
28
If Klydon fails to perform its obligations under the Turnkey Contract, we would be required to build and develop our internal capabilities more quickly than anticipated, and it is possible that we will not be able to do so within the time needed to operate our business effectively.
If we use hazardous and chemical materials in a manner that causes injury or violates applicable law, we may be liable for damages.
Our research and development activities involve the controlled use of potentially hazardous substances, including chemical materials. Klydon is subject to international and local laws and regulations in South Africa governing the use, manufacture, storage, handling and disposal of radioactive and hazardous materials. Although we believe that Klydon’s procedures for using, handling, storing and disposing of these materials comply with legally prescribed standards, we cannot completely eliminate the risk of contamination or injury resulting from radioactive or hazardous materials. As a result of any such contamination or injury, we may incur liability or local, city, state or federal authorities may curtail the use of these materials and interrupt our business operations. In the event of an accident, we could be held liable for damages or penalized with fines, and the liability could exceed our resources. We do not have any insurance for liabilities arising from radioactive or hazardous materials. Compliance with applicable environmental laws and regulations is expensive, and current or future environmental regulations may impair our research, development, and production efforts, which could harm our business, prospects, financial condition, or results of operations.
Risks Related to Our Business Operations, Employee Matters and Managing Growth
We are highly dependent on the services of our senior management team and if we are not able to retain these members of our management team and recruit and retain additional management, clinical and scientific personnel, our business will be harmed.
We are highly dependent on our senior management team. The employment agreements we have with these officers do not prevent such persons from terminating their employment with us at any time. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
In addition, we will need to attract, retain and motivate highly qualified additional management and scientific personnel. If we are not able to retain our management and to attract, on acceptable terms, additional qualified personnel necessary for the continued development of our business, we may not be able to sustain our operations or grow.
We may not be able to attract or retain qualified personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses. Many of the other pharmaceutical companies that we compete against for qualified personnel and consultants have greater financial and other resources, different risk profiles and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates and consultants than what we have to offer. If we are unable to continue to attract, retain and motivate high-quality personnel and consultants to accomplish our business objectives, the rate and success at which we can develop future isotopes and our business will be limited and we may experience constraints on our development objectives.
Our future performance will also depend, in part, on our ability to successfully integrate newly hired executive officers into our management team and our ability to develop an effective working relationship among senior management. Our failure to integrate these individuals and create effective working relationships among them and other members of management could result in inefficiencies in the development and commercialization of our future isotopes, harming future regulatory approvals, sales of our future isotopes and our results of operations. Additionally, we do not currently maintain “key person” life insurance on the lives of our executives or any of our employees.
We will need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations.
As of September 30, 2022, we had four full-time employees. We currently operate as a virtual company and rely on service providers for certain general administrative, financial, accounting, tax, intellectual property and other legal services, and we will need to expand our organization to hire qualified personnel to perform these functions
29
internally. Our management may need to divert significant attention and time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational inefficiencies, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of our future isotopes. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and grow revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance, our ability to commercialize future isotopes, develop a scalable infrastructure and compete effectively will depend, in part, on our ability to effectively manage any future growth.
Our employees, consultants and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, consultants and commercial partners. Misconduct by these parties could include intentional failures to comply with FDA regulations or the regulations applicable in other jurisdictions, provide accurate information to the FDA and other regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from government investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could have a negative impact on our business, financial condition, results of operations and prospects, including the imposition of significant fines or other sanctions.
Significant disruptions of our information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us.
We are dependent on information technology systems and infrastructure, including mobile technologies, to operate our business. In the ordinary course of our business, we collect, store, process and transmit large amounts of sensitive information, including intellectual property, proprietary business information, personal information and other confidential information. It is critical that we do so in a secure manner to maintain the confidentiality, integrity and availability of such sensitive information. We have also outsourced elements of our operations (including elements of our information technology infrastructure) to third parties, and as a result, we manage a number of third-party vendors who may or could have access to our computer networks or our confidential information. In addition, many of those third parties in turn subcontract or outsource some of their responsibilities to third parties. While all information technology operations are inherently vulnerable to inadvertent or intentional security breaches, incidents, attacks and exposures, the accessibility and distributed nature of our information technology systems, and the sensitive information stored on those systems, make such systems potentially vulnerable to unintentional or malicious, internal and external attacks on our technology environment. In addition, all of our employees work remotely, which may make us more vulnerable to cyberattacks. Potential vulnerabilities can be exploited from inadvertent or intentional actions of our employees, third-party vendors, business partners, or by malicious third parties. Attacks of this nature are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives (including, but not limited to, industrial espionage) and expertise, including organized criminal groups, “hacktivists,” nation states and others. In addition to the extraction of sensitive information, such attacks could include the deployment of harmful malware, ransomware, denial-of-service attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information. In addition, the prevalent use of mobile devices increases the risk of data security incidents.
Significant disruptions of our, our third-party vendors’ and/or our business partners’ information technology systems or other similar data security incidents could adversely affect our business operations and/or result in the loss, misappropriation, and/or unauthorized access, use or disclosure of, or the prevention of access to, sensitive information, which could result in financial, legal, regulatory, business and reputational harm to us. In addition, information technology system disruptions, whether from attacks on our technology environment or from computer viruses, natural disasters, terrorism, war and telecommunication and electrical failures, could result in a material disruption of our development programs and our business operations. Additionally, theft of our intellectual property or proprietary business information could require substantial expenditures to remedy. If we or our third-party
30
collaborators, consultants, contractors, suppliers, or service providers were to suffer an attack or breach, for example, that resulted in the unauthorized access to or use or disclosure of personal or health information, we may have to notify consumers, partners, collaborators, government authorities, and the media, and may be subject to investigations, civil penalties, administrative and enforcement actions, and litigation, any of which could harm our business and reputation.
There is no way of knowing with certainty whether we have experienced any data security incidents that have not been discovered. While we have no reason to believe this to be the case, attackers have become very sophisticated in the way they conceal access to systems, and many companies that have been attacked are not aware that they have been attacked. Any event that leads to unauthorized access, use or disclosure of personal information, including but not limited to personal information regarding our patients or employees, could disrupt our business, harm our reputation, compel us to comply with applicable federal and/or state breach notification laws and foreign law equivalents, subject us to time consuming, distracting and expensive litigation, regulatory investigation and oversight, mandatory corrective action, require us to verify the correctness of database contents, or otherwise subject us to liability under laws, regulations and contractual obligations, including those that protect the privacy and security of personal information. This could result in increased costs to us, and result in significant legal and financial exposure and/or reputational harm. In addition, any failure or perceived failure by us or our vendors or business partners to comply with our privacy, confidentiality or data security-related legal or other obligations to third parties, or any further security incidents or other inappropriate access events that result in the unauthorized access, release or transfer of sensitive information, which could include personally identifiable information, may result in governmental investigations, enforcement actions, regulatory fines, litigation, or public statements against us by advocacy groups or others, and could cause third parties, including clinical sites, regulators or current and potential partners, to lose trust in us or we could be subject to claims by third parties that we have breached our privacy- or confidentiality-related obligations, which could materially and adversely affect our business and prospects. Moreover, data security incidents and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above. While we have implemented security measures intended to protect our information technology systems and infrastructure, there can be no assurance that such measures will successfully prevent service interruptions or security incidents.
Our international operations subject us to risks of doing business in foreign countries, which could adversely affect our business, financial condition and results of operations.
Our primary operations are located outside the U.S. (primarily the construction of the isotope enrichment plant in South Africa) and we plan to sell our isotopes to customers outside the U.S. Accordingly, our business is subject to risks related to the differing legal, political, social and regulatory requirements and economic conditions of non-U.S. jurisdictions. Risks inherent in international operations include the following:
• fluctuations in foreign currency exchange rates may affect product demand and may adversely affect the profitability in U.S. dollars of products and services we provide in international markets where payment for our products and services is made in the local currency;
• transportation and other shipping costs may increase, or transportation may be inhibited;
• increased cost or decreased availability of raw materials;
• changes in foreign laws and tax rates or U.S. laws and tax rates with respect to foreign income may unexpectedly increase the rate at which our income is taxed, impose new and additional taxes on remittances, repatriation or other payments by subsidiaries, or cause the loss of previously recorded tax benefits;
• foreign countries in which we do business may adopt other restrictions on foreign trade or investment, including currency exchange controls;
• trade sanctions by or against these countries could result in our losing access to customers and suppliers in those countries;
31
• unexpected adverse changes in foreign laws or regulatory requirements may occur;
• our agreements with counterparties in foreign countries may be difficult for us to enforce and related receivables may be difficult for us to collect;
• compliance with the variety of foreign laws and regulations may be unduly burdensome;
• compliance with anti-bribery and anti-corruption laws (such as the Foreign Corrupt Practices Act) as well as anti-money- laundering laws may be costly;
• unexpected adverse changes in export duties, quotas and tariffs and difficulties in obtaining export licenses may occur;
• general economic conditions in the countries in which we operate could have an adverse effect on our earnings from operations in those countries;
• our foreign operations may experience staffing difficulties and labor disputes;
• termination or substantial modification of international trade agreements may adversely affect our access to raw materials and to markets for our products outside the U.S.;
• foreign governments may nationalize or expropriate private enterprises;
• increased sovereign risk (such as default by or deterioration in the economies and credit worthiness of local governments) may occur; and
• political or economic repercussions from terrorist activities, including the possibility of hyperinflationary conditions and political instability, may occur in certain countries in which we do business.
Unanticipated events, such as geopolitical changes, could result in a write-down of our investment in the affected joint venture or a delay or cause cancellation of those capital projects, which could negatively impact our future growth and profitability. Our success as a global business will depend, in part, upon our ability to succeed in differing legal, regulatory, economic, social and political conditions by developing, implementing and maintaining policies and strategies that are effective in each location where we and our joint ventures do business.
Furthermore, we will be subject to rules and regulations related to anti-bribery and anti-trust prohibitions of the U.S. and other countries, as well as export controls and economic embargoes, violations of which may carry substantial penalties. For example, export control and economic embargo regulations limit the ability of our subsidiaries to market, sell, distribute or otherwise transfer their products or technology to prohibited countries or persons. Failure to comply with these regulations could subject our subsidiaries to fines, enforcement actions and/or have an adverse effect on our reputation and the value of our common stock.
Our tangible assets may be subject to defects in title.
We have investigated our rights to the assets we have purchased and developed and, to the best of our knowledge, those rights are in good standing. However, no assurance can be given that such rights will not be revoked, or significantly altered, to our detriment. There can also be no assurance that our rights will not be challenged or impugned by third parties, including by governments, and non-governmental organizations.
We are subject to foreign currency risks.
Our operations are subject to foreign currency fluctuations. Our operating expenses and revenues are primarily transacted in U.S. dollars, while some of our cash balances and expenses are measured in other currencies. Any strengthening or weakening of the U.S. dollar in relation to the currencies of other countries or vice versa can have a material impact on our cash flows and profitability and affect the value of our assets and shareholders’ equity.
32
Risks Related to This Offering and Ownership of Our Common Stock
We do not know whether an active, liquid and orderly trading market will develop for our common stock or what the market price of our common stock will be and as a result it may be difficult for you to sell your shares of our common stock.
Prior to this offering there has been no public market for shares of our common stock. Although we have applied to list our common stock on the Nasdaq Capital Market (Nasdaq), an active trading market for our shares may never develop or be sustained following this offering. You may not be able to sell your shares quickly or at the market price if trading in shares of our common stock is not active. The initial public offering price for our common stock will be determined through negotiations with the underwriters, and the negotiated price may not be indicative of the market price of our common stock after the offering. As a result of these and other factors, you may be unable to resell your shares of our common stock at or above the initial public offering price.
Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic partnerships or acquire companies or products by using our shares of common stock as consideration.
The price of our stock may be volatile, and you could lose all or part of your investment.
The trading price of our common stock following this offering may be subject to instances of extreme stock price run-ups followed by rapid price declines and stock price volatility unrelated to both our actual and expected operating performance and financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our stock. Further, the trading price of our common stock following this offering is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including limited trading volume.
In addition to the factors discussed in this “Risk Factors” section and elsewhere in this prospectus, these factors include:
• adverse results or delays in our development activities;
• adverse regulatory decisions, including failure to receive regulatory approval for our future isotopes;
• changes in laws or regulations applicable to our future isotopes, including but not limited to requirements for approvals;
• any changes to our relationship with any manufacturers, suppliers, licensors, future collaborators or other strategic partners;
• our inability to obtain adequate product supply for any future isotope or inability to do so at acceptable prices;
• our inability to establish collaborations if needed;
• our failure to commercialize our future isotopes;
• additions or departures of key scientific or management personnel;
• unanticipated serious safety concerns related to the use of our future isotopes;
• introduction of new products or services offered by us or our competitors;
• announcements of significant acquisitions, strategic partnerships, joint ventures, or capital commitments by us or our competitors;
• our ability to effectively manage our growth;
• actual or anticipated variations in quarterly operating results;
• our cash position;
33
• our failure to meet the estimates and projections of the investment community or that we may otherwise provide to the public;
• publication of research reports about us or our industry or positive or negative recommendations or withdrawal of research coverage by securities analysts;
• changes in the market valuations of similar companies;
• overall performance of the equity markets;
• issuances of debt or equity securities;
• sales of our common stock by us or our stockholders in the future or the perception that such sales may occur;
• trading volume of our common stock;
• changes in accounting practices;
• ineffectiveness of our internal controls;
• disputes or other developments relating to proprietary rights, including patents, litigation matters, and our ability to obtain patent protection for our technologies;
• significant lawsuits, including patent or stockholder litigation;
• general political and economic conditions, including the COVID-19 pandemic; and
• other events or factors, many of which are beyond our control.
In addition, the stock market in general, and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. If the market price of our common stock after this offering does not exceed the initial public offering price, you may not realize any return on your investment in us and may lose some or all of your investment. In the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. This type of litigation, if instituted, could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results or financial condition.
We do not intend to pay dividends on our common stock so any returns will be limited to the value of our stock.
We have never declared or paid any cash dividend on our common stock. We currently anticipate that we will retain future earnings for the development, operation, and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to stockholders would therefore be limited to the appreciation, if any, of their stock.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
Prior to this offering, our executive officers, current directors, greater than 5% holders, and their affiliates beneficially owned approximately 35.8% of our common stock as of September 30, 2022. Upon the closing of this offering, that same group will hold approximately 34.1% of our outstanding common stock, assuming the sale of 1,500,000 shares of common stock in this offering (based on shares of common stock outstanding as of September 30, 2022 and excluding awards of 3,000,000 shares of restricted stock that we anticipate making upon the effectiveness of the registration statement of which this prospectus is a part). Therefore, even after this offering, these stockholders will have the ability to influence us through this ownership position. These stockholders may be able to determine all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders.
34
If you purchase our common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
The initial public offering price is substantially higher than the net tangible book value per share of our common stock. Investors purchasing common stock in this offering will pay a price per share that substantially exceeds the book value of our tangible assets after subtracting our liabilities. As a result, investors purchasing common stock in this offering will incur immediate dilution of $4.10 per share, based on the assumed initial public offering price of $4.50 per share and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Further, investors purchasing common stock in this offering will contribute approximately 6.2% of the total amount invested by stockholders since our inception, but will own only approximately 4.7% of the shares of common stock outstanding after giving effect to this offering.
This dilution is due to our investors who purchased shares prior to this offering having paid substantially less when they purchased their shares than the price offered to the public in this offering. To the extent outstanding options are exercised, there will be further dilution to new investors. As a result of the dilution to investors purchasing shares in this offering, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation. For a further description of the dilution that you will experience immediately after this offering, see the section entitled “Dilution.”
Sales of a substantial number of shares of our common stock by our existing stockholders in the public market, or the perception that such sales could occur, could cause our stock price to fall.
If and when the selling stockholders and our other existing stockholders sell, or indicate an intention to sell, substantial amounts of our common stock in the public market after the lock-up and other legal restrictions on resale discussed in this prospectus lapse, the trading price of our common stock could decline. Based on shares of common stock outstanding as of September 30, 2022, upon the closing of this offering we will have outstanding a total of 31,607,127 shares of common stock. Of these shares, only the shares of common stock sold in this offering by us, plus any shares sold upon exercise of the underwriters’ option to purchase additional shares, and the selling stockholder shares that are being registered in the registration statement of which this prospectus is a part, will be freely tradable without restriction in the public market immediately following this offering.
Subject to the restrictions described in the paragraph below, future sales in the public market of shares issued prior to this offering will be subject to the volume and other restrictions of Rule 144 under the Securities Act for so long as they are held by a person that is deemed to be our affiliate, unless the shares to be sold are registered with the SEC. The sale of a substantial number of shares after this offering, or a perception that such sales could occur, could significantly reduce the market price of our common stock.
We expect that the lock-up agreements pertaining to this offering will expire 180 days from the date of this prospectus. After the lock-up agreements expire, up to an additional 28,711,294 shares of common stock will be eligible for sale in the public market, of which 14,376,125 shares are held by directors, executive officers, and other affiliates and will be subject to volume limitations under Rule 144 under the Securities Act. In addition, 7,901,000 shares of common stock that are either subject to outstanding options or reserved for future issuance under our employee benefit plans will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements, and Rule 144 and Rule 701 under the Securities Act. If these additional shares of common stock are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
After this offering, the holders of 3,012,280 shares of our common stock will be entitled to rights with respect to the registration of their shares under the Securities Act, subject to the 180-day lock-up agreements described above. See the section entitled “Description of Capital Stock — Registration Rights.” Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by affiliates, as defined in Rule 144 under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
35
Participation in this offering by certain of our existing stockholders and their affiliated entities may reduce the public float for our common stock.
If any of our existing stockholders and their affiliated entities purchase shares of our common stock in this offering, such purchases would reduce the available public float of our common stock because such purchasers would be restricted from selling such shares during the 180-day period following this offering and thereafter would be subject to volume limitations pursuant to restrictions under applicable securities laws. As a result, any purchase of shares of our common stock by our existing stockholders and their affiliated entities in this offering will reduce the liquidity of our common stock relative to what it would have been had these shares been purchased by investors that were not our stockholders.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that we will need significant additional capital in the future to continue our planned operations, including development activities, commercialization efforts if we are able to obtain marketing approval of future isotopes, research and development activities, and costs associated with operating a public company. To raise capital, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to the holders of our common stock, including shares of common stock sold in this offering.
Pursuant to our 2022 Plan, our management is authorized to grant stock options to our employees, directors and consultants. Additionally, the number of shares of our common stock reserved for issuance under our 2022 Plan will automatically increase on January 1 of each year, beginning on January 1, 2023 and continuing through and including January 1, 2032, by 5% of the total number of shares of our capital stock outstanding on December 31 of the preceding calendar year (determined on an as-converted to voting common stock basis, without regard to any limitations on the conversion of the non-voting common stock), or a lesser number of shares determined by our board of directors.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our net proceeds in ways that ultimately increase the value of your investment. The failure by our management to apply these funds effectively could harm our business. We intend to invest the net proceeds to us from the offering that are not used as described above in short- and medium-term, investment-grade, interest-bearing instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
We are an emerging growth company and a smaller reporting company, and the reduced reporting requirements applicable to emerging growth companies and smaller reporting companies may make our common stock less attractive to investors.
We are an emerging growth company, as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding nonbinding advisory votes on executive compensation and stockholder approval of any
36
golden parachute payments not previously approved. We could be an emerging growth company until December 31, 2027, although circumstances could cause us to lose that status earlier, including if we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act or if we have total annual gross revenue of $1.07 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in non-convertible debt during any three year period before that time, we would cease to be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” which would allow us to take advantage of many of the same exemptions from disclosure requirements including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. Investors may find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to delay the adoption of accounting standards that have different effective dates for public and private companies until those standards would otherwise apply to private companies. We have elected to avail ourselves of this exemption from new or revised accounting standards, and therefore we will not be subject to the same requirements to adopt new or revised accounting standards as other public companies that are not emerging growth companies.
We are also a “smaller reporting company” as defined in the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as our voting and non-voting common stock held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter, or our annual revenue is less than $100.0 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
Delaware law and provisions in our amended and restated certificate of incorporation and amended and restated bylaws that will be in effect at the closing of this offering could make a merger, tender offer or proxy contest difficult, thereby depressing the trading price of our common stock.
Provisions of our amended and restated certificate of incorporation and amended and restated bylaws, which will become effective upon the closing of this offering, may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, our amended and restated certificate of incorporation and amended and restated bylaws will:
• permit our board of directors to issue up to 10,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate (including the right to approve an acquisition or other change in our control);
• provide that the authorized number of directors may be changed only by resolution of the board of directors;
• provide that our board of directors or any individual director may only be removed with cause and the affirmative vote of the holders of at least 66-2/3% of the voting power of all of our then-outstanding shares of the capital stock entitled to vote generally in the election of directors, voting together as a single class;
• provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
37
• divide our board of directors into three classes;
• require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent subject to the rights of the holders of any series of preferred stock;
• provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner and also specify requirements as to the form and content of a stockholder’s notice;
• do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose);
• provide that special meetings of our stockholders may be called only by the chair of our board of directors, our Chief Executive Officer or by the board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors; and
• provide that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for the following types of actions or proceedings under state, statutory and common law: (i) any derivative action or proceeding brought on our behalf; (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders; (iii) any action asserting a claim pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our bylaws; (iv) any action as to which the Delaware General Corporation Law confers jurisdiction to the Court of Chancery of the State of Delaware; and (vi) any action governed by the internal affairs doctrine, in all cases subject to the court’s having personal jurisdiction over the indispensable parties named as defendants; provided these provisions of our amended and restated certificate of incorporation and amended and restated bylaws will not apply to suits brought to enforce a duty or liability created by the Exchange Act, or any other claim for which the federal courts have exclusive jurisdiction; and provided that, unless we consent in writing to the selection of an alternative forum, to the fullest extent permitted by law, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act of 1933, as amended (Securities Act), including all causes of action asserted against any defendant to such complaint. For the avoidance of doubt, this provision is intended to benefit and may be enforced by us, our officers and directors, the underwriters to any offering giving rise to such complaint, and any other professional or entity whose profession gives authority to a statement made by that person or entity and who has prepared or certified any part of the documents underlying the offering.
The amendment of any of these provisions, with the exception of the ability of our board of directors to issue shares of preferred stock and designate any rights, preferences and privileges thereto, would require approval by the holders of at least 66-2/3% of our then-outstanding common stock.
In addition, as a Delaware corporation, we are subject to Section 203 of the Delaware General Corporation Law. These provisions may prohibit large stockholders, in particular those owning 15% or more of our outstanding voting stock, from merging or combining with us for a certain period of time. A Delaware corporation may opt out of this provision by express provision in its original certificate of incorporation or by amendment to its certificate of incorporation or bylaws approved by its stockholders. However, we have not opted out of this provision.
These and other provisions in our amended and restated certificate of incorporation, amended and restated bylaws and Delaware law could make it more difficult for stockholders or potential acquirors to obtain control of our board of directors or initiate actions that are opposed by our then-current board of directors, including delay or impede a merger, tender offer or proxy contest involving our company. The existence of these provisions could negatively affect the price of our common stock and limit opportunities for you to realize value in a corporate transaction.
For information regarding these and other provisions, see “Description of Capital Stock.”
38
Our amended and restated certificate of incorporation will provide that the Court of Chancery of the State of Delaware will be the exclusive forum for certain disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our amended and restated certificate of incorporation that will become effective upon the closing of this offering will provide that, subject to the court’s having personal jurisdiction over the indispensable parties named as defendants, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for the following types of actions or proceedings:
• any derivative action or proceeding brought on our behalf;
• any action asserting a claim of breach of fiduciary duty owed by any of our directors, officers or other employees to us or our stockholders;
• any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or bylaws;
• any action as to which the Delaware General Corporation Law confers jurisdiction to the Court of Chancery of the State of Delaware; and
• any action asserting a claim that is governed by the internal affairs doctrine.
This provision would not apply to suits brought to enforce a duty or liability created by the Exchange Act. Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly, both state and federal courts have jurisdiction to entertain such claims. To prevent having to litigate claims in multiple jurisdictions and the threat of inconsistent or contrary rulings by different courts, among other considerations, our amended and restated certificate of incorporation further provides that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions. In such instance, we would expect to vigorously assert the validity and enforceability of the exclusive forum provisions of our amended and restated certificate of incorporation. This may require significant additional costs associated with resolving such action in other jurisdictions and there can be no assurance that the provisions will be enforced by a court in those other jurisdictions.
These exclusive forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage lawsuits against us and our directors, officers and other employees. If a court were to find either exclusive forum provision in our amended and restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur further significant additional costs associated with resolving the dispute in other jurisdictions, all of which could seriously harm our business.
General Risk Factors
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting, and other expenses that we did not incur as a private company. We will be subject to the reporting requirements of the Exchange Act, which will require, among other things, that we file with the SEC annual, quarterly, and current reports with respect to our business and financial condition. In addition, the Sarbanes-Oxley Act, as well as rules subsequently adopted by the SEC and Nasdaq to implement provisions of the Sarbanes-Oxley Act, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Further, in July 2010, the Dodd-Frank Wall Street Reform and Consumer Protection Act (Dodd-Frank Act) was enacted. There are significant corporate governance and executive compensation related
39
provisions in the Dodd-Frank Act that require the SEC to adopt additional rules and regulations in these areas such as “say on pay” and proxy access. Emerging growth companies and smaller reporting companies are exempted from certain of these requirements, but we may be required to implement these requirements sooner than budgeted or planned and thereby incur unexpected expenses. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
We expect the rules and regulations applicable to public companies to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. If these requirements divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business, financial condition, and results of operations. The increased costs will decrease our net income or increase our net loss, and may require us to reduce costs in other areas of our business or increase the prices of our products or services. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
We have identified a material weakness in our internal control over financial reporting. If our remediation of this material weakness is not effective, or if we experience material weaknesses in the future or otherwise fail to implement and maintain an effective system of internal controls in the future, we may not be able to accurately report our financial condition or results of operations which may adversely affect investor confidence in us, and as a result, the value of our common stock.
As a privately-held company, we were not required to evaluate our internal control over financial reporting in a manner that meets the standards of publicly traded companies required by Section 404(a) of the Sarbanes-Oxley Act, or Section 404. As a public company, we will be subject to significant requirements for enhanced financial reporting and internal controls. The process of designing and implementing effective internal controls is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company. In addition, we will be required, pursuant to Section 404, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting in the second annual report following the completion of this offering. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency or combination of deficiencies in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual and interim financial statements will not be detected or prevented on a timely basis.
The rules governing the standards that must be met for our management to assess our internal control over financial reporting are complex and require significant documentation, testing, and possible remediation. Testing and maintaining internal controls may divert management’s attention from other matters that are important to our business. Once we are no longer an “emerging growth company,” or a “smaller reporting company”, our auditors will be required to issue an attestation report on the effectiveness of our internal controls on an annual basis.
In the course of preparing the financial statements that are included in this prospectus, management has determined that a material weakness exists within the internal controls over financial reporting. The material weakness identified relates to the lack of a sufficient complement of personnel within the finance and accounting function with an appropriate degree of knowledge, experience and training. We also noted a material weakness related to logical security and privileged access in the area of information technology. We concluded that the material weakness in our internal control over financial reporting occurred because, prior to this offering, we were a private company and did not have the necessary business processes, systems, personnel, and related internal controls necessary to satisfy the accounting and financial reporting requirements of a public company.
40
In order to remediate the material weakness, we expect to hire additional accounting and finance resources or consultants with public company experience.
We may not be able to fully remediate the identified material weakness until the steps described above have been completed and our internal controls have been operating effectively for a sufficient period of time. We believe we have already and will continue to make progress in our remediation plan during the year ending December 31, 2022, but cannot assure you that we will be able to fully remediate the material weakness by such time. If the steps we take do not correct the material weakness in a timely manner, we will be unable to conclude that we maintain effective internal control over financial reporting. Accordingly, there could continue to be a reasonable possibility that a material misstatement of our financial statements would not be prevented or detected on a timely basis. We also may incur significant costs to execute various aspects of our remediation plan but cannot provide a reasonable estimate of such costs at this time.
In accordance with the provisions of the JOBS Act, we and our independent registered public accounting firm were not required to, and did not, perform an evaluation of our internal control over financial reporting as of December 31, 2021 nor any period subsequent in accordance with the provisions of the Sarbanes-Oxley Act. Accordingly, we cannot assure you that we have identified all material weaknesses. Material weaknesses may still exist when we report on the effectiveness of our internal control over financial reporting as required under Section 404 of the Sarbanes-Oxley Act after the completion of this offering.
In the future, it is possible that additional material weaknesses or significant deficiencies may be identified that we may be unable to remedy before the requisite deadline for these reports. Our ability to comply with the annual internal control reporting requirements will depend on the effectiveness of our financial reporting and data systems and controls across our company. Any weaknesses or deficiencies or any failure to implement new or improved controls, or difficulties encountered in the implementation or operation of these controls, could harm our operating results and cause us to fail to meet our financial reporting obligations, or result in material misstatements in our consolidated financial statements, which could adversely affect our business and reduce our stock price.
If we are unable to conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404, our independent registered public accounting firm may not issue an unqualified opinion. If we are unable to conclude that we have effective internal control over financial reporting, investors could lose confidence in our reported financial information, which could have a material adverse effect on the trading price of our common stock. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biopharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
Our failure to meet Nasdaq’s continued listing requirements could result in a delisting of our common stock.
If, after listing, we fail to satisfy the continued listing requirements of Nasdaq, such as the corporate governance requirements or the minimum closing bid price requirement, Nasdaq may take steps to delist our common stock. Such a delisting would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we can provide no assurance that any action taken by us to restore compliance with listing requirements would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with the listing requirements of Nasdaq.
41
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on our company. If no securities or industry analysts commence coverage of our company, the trading price for our stock would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who covers us downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price may decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
42
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of the federal securities laws that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations or financial condition, business strategy and plans, and objectives of management for future operations are forward-looking statements. In some cases, you can identify forward-looking statements because they contain words such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “would,” “potential” or “continue” or the negative of these words or other similar terms or expressions that concern our expectations, strategy, plans or intentions. These forward-looking statements include, among others, statements relating to our future financial performance, our business prospects and strategy, our market opportunity and the potential growth of that market, our anticipated financial position, our liquidity and capital needs and other similar matters. These forward-looking statements are based on management’s current expectations and assumptions about future events, which are inherently subject to uncertainties, risks and changes in circumstances that are difficult to predict.
Our actual results may differ materially from those expressed in, or implied by, the forward-looking statements included in this prospectus as a result of various factors, including, among others:
• our ability to complete and commission the Mo-100 enrichment plant in a timely and cost-effective manner, and our dependence upon Klydon for the completion and commissioning of the Mo-100 enrichment plant;
• our ability to meet, and to continue to meet, applicable regulatory requirements for the use of the isotopes we may produce using the ASP technology;
• our ability to comply on an ongoing basis with the numerous regulatory requirements applicable to the ASP technology and our M0-100 enrichment facility in South Africa;
• the introduction, market acceptance and success of Mo-100 that we may produce using ASP technology as an alternative and potentially more convenient production route for Tc-99m;
• the success or profitability of our future offtake arrangements with respect to Mo-100 that we may produce using ASP technology;
• a failure of demand for Mo-100 that we may produce using ASP technology;
• our future capital requirements and sources and uses of cash;
• our ability to obtain funding for its operations and future growth;
• the extensive costs, time and uncertainty associated with new technology development;
• developments and projections relating to our competitors and industry;
• the ability to recognize the anticipated benefits of the acquisition of assets of Molybdos (Pty) Limited in the “business rescue” auction and the ASP technology for the production of Mo-100 and U-235 we licensed from Klydon Proprietary Ltd;
• problems with the performance of the ASP technology in the enrichment of isotopes;
• our dependence on a limited number of third party suppliers for certain components;
• our inability to adapt to changing technology and diagnostic landscape, such as the emergence of new diagnostic scanners or tracers;
• our expected dependence on a limited number of key customers for Mo-100 that we may produce using ASP technology;
• our inability to protect our intellectual property and the risk of claims that we have infringed on the intellectual property of others;
43
• our inability to compete effectively;
• risks associated with the current economic environment;
• risks associated with our international operations;
• geopolitical risk and changes in applicable laws or regulations;
• our inability to adequately protect our technology infrastructure;
• our inability to hire or retain skilled employees and the loss of any of our key personnel;
• operational risk;
• costs and other risks associated with becoming a reporting company and becoming subject to the Sarbanes-Oxley Act;
• our inability to implement and maintain effective internal controls; and
• other factors that are described in “Risk Factors,” beginning on page 9.
We have based the forward-looking statements contained in this prospectus primarily on our current expectations and projections about future events and trends that we believe may affect our business, financial condition, results of operations, prospects, business strategy and financial needs. The outcome of the events described in these forward-looking statements is subject to risks, uncertainties, assumptions and other factors described in the section captioned “Risk Factors” and elsewhere in this prospectus. These risks are not exhaustive. Other sections of this prospectus include additional factors that could adversely impact our business and financial performance. Furthermore, new risks and uncertainties emerge from time to time and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this prospectus. The results, events and circumstances reflected in the forward-looking statements may not be achieved or occur, and actual results, events or circumstances could differ materially from those described in the forward-looking statements.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this prospectus, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement of which this prospectus forms a part with the understanding that our actual future results, levels of activity, performance and achievements may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
The forward-looking statements made in this prospectus relate only to events as of the date on which such statements are made. We undertake no obligation to update any forward-looking statements after the date of this prospectus or to conform such statements to actual results or revised expectations, except as required by law.
44
Market, Industry and Other Data
We use market and industry data, forecasts and projections throughout this prospectus. We have obtained certain market and industry data from publicly available industry publications and from certain sources that are not publicly available. These sources generally state that the information they provide has been obtained from sources believed to be reliable, but that the accuracy and completeness of the information are not guaranteed. The forecasts and projections are based on historical market data, and any of the forecasts or projected amounts may not be achieved. The market and industry data used in this prospectus involve risks and uncertainties that are subject to change based on various factors, including those discussed in the section titled “Risk Factors.” These and other factors could cause results to differ materially from those expressed in, or implied by, the estimates made by independent parties and by us. Furthermore, we cannot assure you that a third party using different methods to assemble, analyze or compute industry and market data would obtain the same results.
45
We estimate that the net proceeds to us from the sale of shares of our common stock in this offering will be approximately $5.4 million, based upon the assumed initial public offering price of $4.50 per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discount and estimated offering expenses payable by us. If the underwriters’ option to purchase additional shares is exercised in full, we estimate that the net proceeds to be received by us will be approximately $6.3 million, after deducting the estimated underwriting discount and estimated offering expenses payable by us. We will not receive any of the proceeds from the sale of selling stockholder shares.
A $1.00 increase (decrease) in the assumed initial public offering price of $4.50 per share would increase (decrease) the net proceeds that we receive from this offering by approximately $1.4 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discount and estimated offering expenses payable by us. Similarly, each increase (decrease) of 1.0 million in the number of shares offered by us would increase (decrease) the net proceeds that we receive from this offering by approximately $4.2 million, assuming that the assumed initial public offering price remains the same and after deducting the estimated underwriting discount and estimated offering expenses payable by us.
The principal purposes of this offering are to increase our capitalization and financial flexibility, create a public market for our common stock and thereby enable access to the public equity markets for us and our stockholders.
We initiated Phase 1 of the Mo-100 development plan under the turnkey contract, targeting 5 kg/year of 95% enriched Mo-100, in October 2021 and we expect to complete this phase using cash on hand during the second half of 2022. Upon completion of Phase 1, we intend to use a portion of the net proceeds from this offering (currently estimated to be approximately $6.0 million of the total net proceeds) to initiate and fully fund Phase 2 of the Mo-100 development plan under the turnkey contract, which targets expanded production of up to 20 kg/year of 95% enriched Mo-100. We intend to use the remainder of the net proceeds we receive from this offering for research and development for potential additional isotopes that we may offer, as well as headcount costs, working capital and other general corporate purposes. We may also use a portion of the net proceeds for acquisitions of, or strategic investments in, complementary businesses, products, services, or technologies. However, we do not have any agreements or commitments to enter into any material acquisitions or investments at this time. Pending their use, we intend to invest the net proceeds from the offering in investment-grade, interest-bearing instruments, such as money market accounts, certificates of deposit, commercial paper and guaranteed obligations of the U.S. government.
This expected use of net proceeds from this offering represents our intentions based on our current plans and business conditions, which could change in the future as our plans and business conditions evolve. We cannot specify with any certainty the particular uses of the net proceeds that we will receive from this offering. Our management will have broad discretion in the application of the net proceeds from this offering and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately.
46
We currently intend to retain all available funds and future earnings, if any, for use in the operation of our business and do not intend to declare or pay any cash dividends in the foreseeable future. Any further determination to pay dividends on our capital stock will be at the discretion of our board of directors, subject to applicable laws, and will depend on our financial condition, results of operations, capital requirements, general business conditions, and other factors that our board of directors consider relevant. Our future ability to pay cash dividends on our common stock may also be limited by the terms of any future debt securities, preferred stock or credit facility.
47
The following table sets forth our cash and cash equivalents and our capitalization as of June 30, 2022, as follows:
• on an actual basis;
• on a pro forma basis to reflect (i) the filing of our Certificate of Incorporation immediately prior to the closing of this offering and (ii) the issuance of 700,000 shares of common stock in July 2022 for no proceeds; and
• on a pro forma as-adjusted basis to give effect to (1) the pro forma item described immediately above, and (2) our issuance and sale of 1,500,000 shares of common stock in this offering at an assumed initial public offering price of $4.50 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discount and our estimated offering expenses.
The pro forma and pro forma as-adjusted information below is illustrative only, and our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this information in conjunction with our consolidated financial statements and the related notes included elsewhere in this prospectus, the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other financial information contained in this prospectus.
|
As of June 30, 2022 |
||||||||||||
|
Actual |
Pro forma |
Pro forma |
||||||||||
|
Cash |
$ |
2,813,411 |
|
$ |
2,813,411 |
|
$ |
8,320,111 |
|
|||
|
|
|
|
|
|
|
|||||||
|
Stockholders’ equity: |
|
|
|
|
|
|
||||||
|
Preferred stock, $0.01 par value; no shares authorized, issued, and outstanding, actual, and 10,000,000 shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted |
$ |
— |
|
$ |
— |
|
$ |
— |
|
|||
|
Common stock, $0.01 par value; 50,000,000 shares authorized, 29,407,127 shares issued and outstanding at June 30, 2022, actual, 500,000,000 shares authorized, 30,107,127 shares issued and outstanding, pro forma; 500,000,000 shares authorized, 31,607,127 shares issued and outstanding, pro forma as adjusted |
|
294,071 |
|
|
301,071 |
|
|
316,071 |
|
|||
|
Additional paid-in capital |
|
11,256,158 |
|
|
12,656,158 |
|
|
17,993,658 |
|
|||
|
Accumulated other comprehensive income |
|
143,994 |
|
|
143,994 |
|
|
143,994 |
|
|||
|
Accumulated deficit |
|
(4,292,685 |
) |
|
(5,692,685 |
) |
|
(5,692,685 |
) |
|||
|
Total stockholders’ equity |
|
7,408,538 |
|
|
7,408,538 |
|
|
12,761,038 |
|
|||
|
Total capitalization |
$ |
7,408,538 |
|
$ |
7,408,538 |
|
$ |
12,761,038 |
|
|||
____________
(1) A $1.00 increase (decrease) in the assumed initial public offering price of our common stock of $4.50 per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, would increase (decrease) the as-adjusted amount of cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $1.4 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discount and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1.0 million in the number of shares offered by us would increase (decrease) the as-adjusted amount of cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $4.2 million, assuming that the assumed initial public offering price remains the same and after deducting the estimated underwriting discount and estimated offering expenses payable by us.
48
If the underwriters’ option to purchase additional shares is exercised in full, pro forma as-adjusted cash and cash equivalents, additional paid-in capital, total stockholders’ equity and total capitalization and shares of common stock outstanding would be $9.3 million, $18.9 million, $13.7 million, $13.7 million and 31,832,127 shares, respectively.
The outstanding share information in the table above is based on 29,407,127 shares of our common stock outstanding as of June 30, 2022 and excludes (1) 2,901,000 shares of our common stock issuable upon the exercise of options outstanding as of September 30, 2022 under the 2021 Plan, at a weighted-average exercise price of $1.91 per share, (2) 5,000,000 shares of common stock reserved for future issuance under the 2022 Plan under which we expect to make all future awards (including awards to our executive officers, directors and consultants of 3,000,000 shares of restricted stock that we anticipate making upon the effectiveness of the registration statement of which this prospectus is a part), and (3) 57,250 shares of common stock issuable to Revere Securities LLC as partial compensation for its role as placement agent in connection with an unregistered offering of shares of common stock.
Our 2022 Plan provides for annual automatic increases in the number of shares reserved thereunder. See the section titled “Executive Compensation — Employee Benefit and Equity Incentive Plans” for additional information.
49
If you invest in our common stock in this offering, your interest will be immediately diluted to the extent of the difference between the initial public offering price per share of our common stock in this offering and the pro forma as-adjusted net tangible book value per share of our common stock after this offering. As of June 30, 2022, we had a historical net tangible book value of $7.3 million, or $0.25 per share of common stock. Our net tangible book value represents total tangible assets less total liabilities, all divided by the number of shares of common stock outstanding on such date.
Our pro forma net tangible book value as of June 30, 2022 was $7.3 million, or $0.24 per share of common stock. Pro forma net tangible book value represents the amount of our total tangible assets less our total liabilities, after giving effect to (i) our issuance of 700,000 shares of common stock in July 2022 for no proceeds. Pro forma net tangible book value per share represents pro forma net tangible book value divided by the total number of shares outstanding as of June 30, 2022, after giving effect to the pro forma adjustment described above.
After giving effect to the sale of 1,500,000 shares of common stock in this offering at an assumed initial public offering price of $4.50 per share, the midpoint of the price range set forth on the cover page of this prospectus and after deducting the estimated underwriting discount and estimated offering expenses payable by us, our pro forma as-adjusted net tangible book value as of June 30, 2022 would have been approximately $12.6 million, or approximately $0.40 per share. This represents an immediate increase in pro forma net tangible book value of $0.16 per share to existing stockholders and an immediate dilution in pro forma net tangible book value of $4.10 per share to new investors purchasing shares of common stock in this offering. Dilution per share to new investors is determined by subtracting pro forma as-adjusted net tangible book value per share after this offering from the initial public offering price per share paid by new investors. The following table illustrates this per share dilution:
|
Assumed initial public offering price per share |
|
|
$ |
4.50 |
|||
|
Historical net tangible book value per share as of June 30, 2022 |
$ |
0.25 |
|
|
|||
|
Decrease per share attributable to the pro forma adjustments described |
|
(0.01 |
) |
|
|
||
|
Pro forma net tangible book value per share as of June 30, 2022, before giving effect to this offering |
|
0.24 |
|
|
|||
|
Increase in historical net tangible book value per share attributable to new investors in this offering |
|
0.16 |
|
|
|
||
|
Pro forma as-adjusted net tangible book value per share immediately after this offering |
|
|
|
0.40 |
|||
|
Dilution per share to new investors in this offering |
|
|
$ |
4.10 |
A $1.00 increase (decrease) in the assumed initial public offering price of $4.50 per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease), our pro forma as-adjusted net tangible book value per share after this offering by $0.04, and would increase (decrease) dilution per share to new investors in this offering by $0.96, in each case assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discount and estimated offering expenses payable by us. Similarly, each increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) our pro forma as-adjusted net tangible book value per share after this offering by approximately $0.12 per share and decrease (increase) the dilution to new investors by approximately $0.12 per share, in each case assuming that the assumed initial public offering price remains the same, and after deducting the estimated underwriting discount and estimated offering expenses payable by us.
Except as otherwise indicated, the above discussion and tables assume no exercise of the underwriters’ option to purchase additional shares. If the underwriters’ option to purchase additional is exercised in full, pro forma as-adjusted net tangible book value after this offering would be approximately $0.43 per share, the increase in pro forma net tangible book value per share to existing stockholders would be $0.19 per share and the dilution per share to new investors would be $4.07 per share, in each case assuming an initial public offering price of $4.50 per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discount and estimated offering expenses payable by us.
50
The following table summarizes, on a pro forma as adjusted basis as of June 30, 2022, the differences between the number of shares of common stock purchased from us, the total consideration paid and the average price per share paid by existing stockholders and to be paid by the new investors purchasing shares of common stock in this offering, at the assumed initial public offering price of common stock of $4.50 per share, the midpoint of the price range set forth on the cover page of this prospectus, before deducting the estimated underwriting discount and estimated offering expenses payable by us.
|
Shares purchased |
Total consideration |
Average price per share |
||||||||||||
|
Number |
Percent |
Amount |
Percent |
|||||||||||
|
Existing investors |
30,107,127 |
95.3 |
% |
$ |
101,537,835 |
93.8 |
% |
$ |
3.37 |
|||||
|
New investors in this offering |
1,500,000 |
4.7 |
|
|
6,750,000 |
6.2 |
|
|
4.50 |
|||||
|
Total |
31,607,127 |
100 |
% |
|
108,287,835 |
100 |
% |
|
||||||
The table above assumes no exercise of the underwriters’ option to purchase additional shares in this offering. If the underwriters’ option to purchase additional shares is exercised in full, the number of shares of our common stock held by existing stockholders would be reduced to 94.6% of the total number of shares of our common stock outstanding after this offering, and the number of shares of common stock held by new investors purchasing common stock in this offering would be increased to 5.4% of the total number of shares of our common stock outstanding after this offering.
The number of shares of our common stock that will be outstanding after this offering is based on 29,407,127 shares of our common stock outstanding as of June 30, 2022, and excludes (1) 2,901,000 shares of our common stock issuable upon the exercise of options outstanding as of September 30, 2022 under the 2021 Plan, at a weighted-average exercise price of $1.91 per share, (2) 5,000,000 shares of common stock reserved for future issuance under the 2022 Plan (including awards to our executive officers, directors and consultants of 3,000,000 shares of restricted stock that we anticipate making upon the effectiveness of the registration statement of which this prospectus is a part), and (3) 57,250 shares of common stock issuable to Revere Securities LLC as partial compensation for its role as placement agent in connection with an unregistered offering of shares of common stock.
Our 2022 Plan provide for annual automatic increases in the number of shares reserved thereunder. See the section titled “Executive Compensation — Employee Benefit and Equity Incentive Plans” for additional information.
To the extent any of the outstanding options are exercised or new options or other securities are issued under our equity incentive plans, you will experience further dilution as a new investor in this offering. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. Furthermore, we may choose to issue common stock as part or all of the consideration in acquisitions as part of our planned growth strategy. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
51
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
RESULTS OF OPERATIONS
The following discussion and analysis of our historical results of operations and our liquidity and capital resources should be read together with the consolidated financial statements and related notes that appear elsewhere in this prospectus. In addition to historical financial information, this prospectus contains “forward-looking statements.” You should review the “Special Note Regarding Forward-Looking Statements” and “Risk Factors” sections of this prospectus for factors and uncertainties that may cause our actual future results to be materially different from those in our forward-looking statements. Forward-looking statements in this prospectus are based on information available to us as of the date hereof, and we assume no obligation to update any such forward-looking statements.
Overview
We are a pre-commercial stage advanced materials company dedicated to the development of technology and processes that, if successful, will allow for the enrichment of natural isotopes into higher concentration products, which could be used in several industries. We were incorporated in Delaware in September 2021 to acquire assets and license intellectual property rights related to the production of isotopes using Aerodynamic Separation Process (“ASP technology”), originally developed and licensed to us by Klydon Proprietary Ltd (“Klydon”). We have an exclusive license to use the ASP technology for the production, distribution, marketing and sale of all isotopes. Our initial focus is on the production and commercialization of enriched Molybdenum-100 (“Mo-100”). Klydon has agreed to provide us a first commercial-scale Mo-100 enrichment plant located in South Africa with a manufacturing capacity of 20 kg/year of 95% enriched Mo-100 when fully operational. We believe that the Mo-100 we may develop using the ASP technology has significant potential advantages for use in the preparation of nuclear imaging agents by radiopharmacies and others in the medical industry. We also intend to use the ASP technology to produce enriched Uranium-235 (“U-235”). We believe that the U-235 we may develop using the ASP technology may be commercialized as a nuclear fuel component for use in the new generation of HALEU-fueled small modular reactors that are now under development for commercial and government uses.
We operate principally through subsidiaries: ASP Isotopes Guernsey Limited (the holding company of ASP Isotopes South Africa (Proprietary) Limited), which will be focused on the development and commercialization of high value, low volume isotopes for highly specialized end markets (such as Mo-100 and others, including Silicon-28); Enriched Energy LLC, which will be focused on the development and commercialization of uranium for the nuclear energy market; and ASP Isotopes UK Ltd, which is the licensee of the ASP technology under the exclusive license agreement with Klydon.
Acquisition of Assets and Agreements with Klydon
To date, we have purchased certain assets of Molybdos Proprietary Limited, a South Africa company (Molybdos), and entered into a number of agreements with Klydon (Pty) Limited, a South Africa company (Klydon). Below is a summary of the key terms for our licenses and other agreements with Klydon. For a more detailed description of these agreements, see the sections of this prospectus entitled “Certain Relationships and Related Party Transactions — Our Relationship with Klydon Proprietary Limited.”
Acquisition of Molybdos Assets. On September 30, 2021, our subsidiary, ASP Isotopes South Africa (Proprietary) Limited (“ASP South Africa”) participated in and was declared the winner of a competitive auction process under Section 45 of the South Africa Consumer Protection Act, 2008 related to the sale and assignment of the assets of Molybdos (the “Molybdos Business Rescue Auction”). On October 12, 2021, ASP South Africa acquired the assets of Molybdos for ZAR 11,000,000 (which at the then current exchange rate was approximately USD734,000), plus value added tax (VAT) levied by the government of South Africa at the rate of 15% and auctioneers’ commission at the rate of 10%.
Acquisition of Silicon-28 Plant Assets. On July 26, 2022, we acquired assets comprising a dormant Silicon-28 aerodynamic separation processing plant from Klydon for ZAR 6,000,000 (which at the then current exchange rate was approximately USD 364,000), which will be payable to Klydon on the later of 180 days of the acquisition and the date on which the assets generate any revenues of any nature.
52
Exclusive Mo-100 License (superseded and replaced by new license (see “Omnibus Klydon License” below)). On September 30, 2021, ASP South Africa, as licensee, entered into a license with Klydon, as licensor, pursuant to which ASP South Africa acquired from Klydon an exclusive license to use, subcontract and sublicense certain intellectual property rights relating to the ASP technology for the development and/or otherwise disposing of the ASP technology and production, distribution, marketing and or sale of Mo-100 isotope produced using the ASP technology (as amended on June 8, 2022, the “Mo-100 license”). The intellectual property rights granted to us through the Mo-100 license included all existing and/or future proprietary rights of Klydon relating to the ASP technology, whether or not such rights have been registered including the copyright, designs, know-how, patents and trademarks (although Klydon currently has no such patents, patent applications or copyrights). The exclusive Mo-100 license was royalty-free, had a term of 999 years and was for the global development of the ASP Technology and production of the Mo-100 Isotope and global for the distribution, marketing and sale of the Mo-100 Isotope. No upfront or other payment was made or is owed in connection with the Mo-100 license. Klydon had the right to terminate the exclusivity of the Mo-100 license in the event that the licensee ceased carrying on activities of Mo-100 enrichment for a period longer than 24 consecutive months. Klydon had no other rights to terminate the Mo-100 license. Effective July 26, 2022, the parties agreed to terminate the Mo-100 license, which was superseded and replaced by a new license agreement (described under the heading “Omnibus Klydon License” below).
Exclusive U-235 License (superseded and replaced by new license (see “Omnibus Klydon License” below)). On January 25, 2022, ASP South Africa, as licensee, entered into a license with Klydon, as licensor, pursuant to which ASP South Africa acquired from Klydon an exclusive license to use, subcontract and sublicense certain intellectual property rights relating to the ASP technology for the development and/or otherwise disposing of the ASP technology and production, distribution, marketing and or sale of U-235 produced using the ASP (as so amended, the “U-235 license”). The intellectual property rights granted to us through the U-235 license included all existing and/or future proprietary rights of Klydon relating to the ASP technology, whether or not such rights have been registered including the copyright, designs, know-how, patents and trademarks (although Klydon currently has no such patents, patent applications or copyrights). The exclusive U-235 license had a term of 999 years and was for the global development of the ASP technology and production of U-235 and global for the distribution, marketing and sale of U-235. In connection with the U-235 license we made an upfront payment of $100,000 and agreed to pay certain royalties (the greater of $50 per k.g. of U-235 and 10% of profits) and a 33% sublicensing revenue share of any cash consideration we may receive for any sublicenses we may grant. Klydon had the right to terminate the exclusivity of the U-235 license in the event that the licensee ceased carrying on activities of U-235 enrichment for a period longer than 24 consecutive months. Klydon had no other rights to terminate the U-235 license. Effective July 26, 2022, the parties agreed to terminate the U-235 license, which was superseded and replaced by a new license agreement (described under the heading “Omnibus Klydon License” below).
Omnibus Klydon License. On July 26, 2022, ASP Isotopes UK Ltd, as licensee, entered into a license agreement with Klydon, as licensor, pursuant to which ASP Isotopes UK Ltd acquired from Klydon an exclusive license to use, develop, modify, improve, subcontract and sublicense certain intellectual property rights relating to the ASP technology for the production, distribution, marketing and sale of all isotopes produced using the ASP technology (the “Klydon license agreement”). The intellectual property rights granted to us through the Klydon license agreement include all existing and/or future proprietary rights of Klydon relating to the ASP technology, whether or not such rights have been registered including the copyright, designs, know-how, patents and trademarks (although Klydon currently has no such patents, patent applications or copyrights). The Klydon license agreement superseded and replaced the Mo-100 license and U-235 license described above. The Klydon license agreement is royalty-free, has a term of 999 years and is worldwide for the development of the ASP technology and the distribution, marketing and sale of isotopes. Future production of isotopes is limited to member countries of the Nuclear Suppliers Group. In connection with the Klydon license agreement we agreed to make an upfront payment of $100,000 (to be included within the payments we make under the Turnkey Contract (described below) and deferred payments of $300,000 over 24 months. Klydon has the right to terminate the exclusivity of the Klydon license agreement in the event that the licensee ceases to carry on activities related to isotope enrichment for a period longer than 24 consecutive months.
Turnkey Contract. On November 1, 2021, ASP South Africa and Klydon, as the contractor, entered into a contract under which Klydon has been appointed to supply to ASP South Africa a complete turnkey Molybdenum-100 enrichment plant (the “Turnkey Contract”). The activities to be undertaken or performed by Klydon include: taking control of the assets acquired in the Molybdos Business Rescue Auction; the design of a Molybdenum-100 enrichment facility with target manufacturing capability of 20 Kg p.a of 95% and above enriched
53
Molybdenum isotope; the supply of components, equipment and labor required for 20 Kg p.a.; the installation, testing and commissioning of the Molybdenum enrichment plant, including production of targets to be used by customers in cyclotrons; securing all required approvals, regulatory authorizations and other required consents for the operation of the plant; providing training to local ASP Isotopes South Africa (Proprietary) Limited personnel to enable them to operate the plant going forward; and providing warranties in relation to the performance targets of the plant which are required to be met. Klydon will be responsible for liaising with the relevant South African authorities including the South African Non Proliferation Council, the Nuclear Suppliers Group and International Atomic Energy Agency to ensure that the Turnkey Contract and the Molybdenum-100 enrichment plant are compliant with international laws and guidelines. The consideration to be paid by ASP Isotopes South Africa (Proprietary) Limited under the Turnkey Contract is a maximum of $12.8 million, in the following stages: (1) $6.8 million in an initial proof of concept stage (which stage will end at the point of first production of Mo-100); and (2) $6.0 million for increasing production capacity through modular construction (from the expected initial capacity of 5 kg p.a. to 20 kg p.a. of 95% enriched molybdenum-100). The Company’s management expects that the initial proof of concept stage (Phase 1) will be completed during the second half of 2022 and an additional 12 months will be needed for completion of the secondary investment stage (Phase 2).
Letter of Intent for Klydon Shares or Assets. On September 30, 2021, ASP South Africa entered into a letter of intent with Klydon and Isotope Separation Technology (Pty) Ltd (Klydon’s largest shareholder which is owned by Dr Ronander and Dr Strydom) with respect to the acquisition of all of the outstanding shares or substantially all of the assets of Klydon. Under the letter of intent (as amended), Klydon has agreed to negotiate with us on an exclusive basis. We are in the process of preparing, and negotiating with Klydon, the share purchase agreement and related agreements with respect to the Klydon acquisition, but such transaction documents are not yet in agreed form and as of the date hereof, several issues remain open that, if not resolved, will prevent us from entering into a definitive agreement with respect to the Klydon acquisition. We do not expect the timing or success of the Klydon acquisition to have a material effect on either our business or our financial results in the future because of the existing commercial agreements that we have with Klydon. We believe that the Klydon license agreement and the Turnkey Contract provide us with the requisite intellectual property rights and personnel (through Klydon’s workforce) that we need to conduct our business as currently proposed to be conducted. While an acquisition of Klydon would be beneficial to us in terms of adding employees in South Africa, the services of the individuals who are working to deliver the Mo-100 enrichment plant are already assured under the Turnkey Contract with Klydon. In the event we do not complete an acquisition of Klydon by the completion of the Turnkey Contract (after Klydon has delivered a fully commissioned Mo-100 enrichment plant), we would likely need to enter into a new agreement with Klydon as a contractor in order to operate the new Mo-100 enrichment plant. Alternatively, we would need to hire employees who would be able to operate the new Mo-100 enrichment plant.
Other Commercial Agreements
Below is a summary of the key terms of our other commercial agreements.
Lease for Molybdenum Processing Plant. On October 12, 2021, ASP South Africa entered into an agreement of lease with the landlord of the facility located at 33 Eland Street, Koedoespoort Industrial, Pretoria where Klydon and its scientists and engineers will operate on our behalf the Molybdenum processing plant where gaseous Molybdenum compound will be treated (which process comprises several stages of compression and expansion during which the product is purified). The term of the lease ends on December 31, 2030.
Political Risk Insurance Policy with Optio Group. On October 25, 2021, ASP Guernsey entered into a contract of insurance to cover against political risk and expropriation, to off-set the risk of events detrimental to the company occurring in the Republic of South Africa for a period of three years. The insurer is Optio Group Limited which is 100% underwritten by one or more syndicates at Lloyd’s of London. The specific risks covered in the policy are: (i) permanent and total abandonment of operations, (ii) deprivation of assets or shareholding, (iii) physical damage due to political violence, (iv) non-transfer or inconvertibility, (v) business interruption, (vi) non-honouring of arbitration award, and (vii) crisis management support. The limit of cover is equal to or in excess of the projected amount of investment required to complete the initial stage of the first planned Molybdenum enrichment plant. The limit of cover is capable of being increased and extended by mutual agreement with the insurer.
54
Components of Results of Operations
Operating Expenses
Our operating expenses consist of (i) research and development expenses and (ii) general and administrative expenses.
Research and Development
Our research and development expenses consist primarily of direct and indirect costs incurred in connection with the development activities for our future isotopes.
Direct costs include:
• external research and development expenses incurred under the Turnkey Contract; and
• costs related to designing the development processes of isotope production.
Indirect costs include:
• personnel-related costs, which include salaries, payroll taxes, employee benefits, and other employee-related costs, including stock-based compensation, for personnel engaged in research and development functions; and
• facilities and other various expenses.
Research and development expenses are recognized as incurred and payments made prior to the receipt of goods or services to be used in research and development are capitalized until the goods or services are received.
As described above, Klydon will charge us for expenses associated with these research and development functions under the Turnkey Contract. We expect that our research and development expenses will increase substantially for the foreseeable future as we continue the development of our future isotopes. We cannot determine with certainty the timing of initiation, the duration or the completion costs of development activities. Actual development timelines, the probability of success and development costs can differ materially from expectations.
We will need to raise substantial additional capital in the future. In addition, we cannot forecast which future isotopes may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
Our research and development expenses may vary significantly based on a variety of factors, such as:
• the scope, rate of progress, expense and results of our development activities;
• the phase of development of our future isotopes;
• the timing, receipt, and terms of any approvals from applicable regulatory authorities including the FDA and foreign regulatory authorities;
• significant and changing government regulation and regulatory guidance;
• the cost and timing of designing the development processes of isotope production;
• the extent to which we establish additional strategic collaborations or other arrangements; and
• the impact of any business interruptions to our operations or to those of the third parties with whom we work, including Klydon, particularly in light of the current COVID-19 pandemic environment.
A change in the outcome of any of these variables with respect to the development of any of our future isotopes could significantly change the costs and timing associated with the development of that future isotope.
55
General and Administrative
General and administrative expenses consist primarily of personnel-related costs, which include salaries, payroll taxes, employee benefits, and other employee-related costs, including stock-based compensation, for personnel in executive, finance and other administrative functions. Other significant costs include legal fees relating to corporate matters, professional fees for accounting and consulting services and facility-related costs.
We expect that our ongoing general and administrative expenses will increase substantially for the foreseeable future to support our increased research and development activities and increased costs of operating as a public company and in building our internal resources. These increased costs will include increased expenses related to audit, legal, regulatory and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and investor and public relations costs associated with operating as a public company.
Results of Operations
Six Months Ended June 30, 2022
The following table summarizes our results of operations for the six months ended June 30, 2022:
|
Six Months |
||||
|
Operating expenses: |
|
|
||
|
Research and development |
$ |
446,440 |
|
|
|
General and administrative |
|
1,239,772 |
|
|
|
Total operating expenses |
|
1,686,212 |
|
|
|
Loss from operations |
|
(1,686,212 |
) |
|
|
Interest Income |
|
1,454 |
|
|
|
Net loss |
$ |
(1,684,758 |
) |
|
Research and Development Expenses
The following table summarizes our research and development expenses for the six months ended June 30, 2022:
|
Six Months |
|||
|
Direct costs: |
|
||
|
Mo-100 |
$ |
6,645 |
|
|
Indirect costs: |
|
||
|
Personnel-related costs |
|
239,688 |
|
|
Consulting, facility and other expenses |
|
200,107 |
|
|
Total research and development expenses |
$ |
446,440 |
|
Research and development expenses were $446,440 for the six months ended June 30, 2022. These expenses include $6,645 in consulting expenses related to advancing development activities for Mo-100, $216,000 of personnel-related costs, $23,688 in stock-based compensation, $99,580 in license fees and $100,527 in consulting, facility and other expenses.
General and Administrative Expenses
General and administrative expenses were $1,239,772 for the six months ended June 30, 2022. These expenses include $397,503 of personnel-related costs, $207,860 in stock-based compensation, $513,333 of professional services and legal related fees and $121,076 in facility and other corporate expenses.
56
Period from September 13, 2021 (Inception) through December 31, 2021
The following table summarizes our results of operations for the period from September 13, 2021 (Inception) through December 31, 2021:
|
For the |
||||
|
Operating expenses: |
|
|
||
|
Research and development |
$ |
41,610 |
|
|
|
General and administrative |
|
2,566,432 |
|
|
|
Total operating expenses |
|
2,608,042 |
|
|
|
Loss from operations |
|
(2,608,042 |
) |
|
|
Interest Income |
|
115 |
|
|
|
Net loss |
$ |
(2,607,927 |
) |
|
Research and Development Expenses
The following table summarizes our research and development expenses for the period from September 13, 2021 (Inception) through December 31, 2021:
|
For the |
|||
|
Direct costs: |
|
||
|
Mo-100 |
$ |
9,360 |
|
|
Indirect costs: |
|
||
|
Facility and related expenses |
|
32,250 |
|
|
Total research and development expenses |
$ |
41,610 |
|
Research and development expenses were $41,610 for the period from September 13, 2021 (Inception) through December 31, 2021. These expenses include $9,360 in consulting expenses related to advancing development activities for Mo-100 and $32,250 in facility and related expenses.
General and Administrative Expenses
General and administrative expenses were $2,566,432 for the period from September 13, 2021 (Inception) through December 31, 2021. These expenses include $1,735,841 of expenses for past services for the issuance of warrants to purchase common shares, $513,227 in stock-based compensation, $127,500 of personnel-related costs, $137,209 of professional services and legal related fees, $21,025 in facility and related expenses and $31,630 in other corporate expenses.
Liquidity and Capital Resources
Sources of Liquidity
We have incurred net losses and negative cash flows from operations since our inception and we expect to continue to incur significant and increasing net losses for the foreseeable future. We have principally financed our operations to date through private placements of our common stock. As of June 30, 2022, we had cash of
57
$2,813,411. We do not have any isotopes approved for sale, we have not generated any revenue from the sale of isotopes, and our ability to generate product revenue sufficient to achieve profitability will depend on the successful development and eventual commercialization of one or more of our current or future isotopes.
Future Funding Requirements
Based on our current operating plan, we estimate that our existing cash, together with the anticipated net proceeds from this offering, will be sufficient to fund our operating expenses and capital expenditure requirements through at least the next 12 months after completion of the offering. However, our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially. We have based this estimate on assumptions that may prove to be wrong, and we could deplete our capital resources sooner than we expect. Additionally, the process of developing isotopes is costly, and the timing of progress and expenses in these development activities is uncertain. Should this offering be delayed or not completed, we will not have sufficient cash resources for the ensuing 12 months.
Our future capital requirements will depend on many factors, including:
• the type, number, scope, progress, expansions, results, costs and timing of, our development activities for our future isotopes;
• the outcome, timing and costs of regulatory review of our future isotopes;
• the costs and timing of manufacturing for our future isotopes;
• our efforts to enhance operational systems and hire additional personnel to satisfy our obligations as a public company, including enhanced internal controls over financial reporting;
• the costs associated with hiring additional personnel and consultants as our preclinical and clinical activities increase;
• the timing and amount of the payments we must make under the Turnkey Contract;
• the costs and timing of establishing or securing sales and marketing and distribution capabilities, whether alone or with third parties, to commercialize future isotopes for which we may obtain regulatory approval, if any;
• our ability to achieve sufficient market acceptance, coverage and adequate reimbursement from third-party payors and adequate market share and revenue for any approved products;
• the terms and timing of establishing and maintaining collaborations, licenses and other similar arrangements;
• the costs of obtaining, expanding, maintaining and enforcing our patent and other intellectual property rights;
• costs associated with any products or technologies that we may in-license or acquire; and
• if we experience any delays or encounter any issues with any of the above, including the risk of each of which may be exacerbated by the ongoing COVID-19 pandemic.
Developing isotopes is a time-consuming, expensive and uncertain process that takes years to complete, and we may never achieve the necessary results required or obtain applicable regulatory approval for any isotopes or generate revenue from the sale of any future isotopes (assuming applicable regulatory approval is received). In addition, our future isotopes (assuming applicable regulatory approval is received) may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of Mo-100 that we do not expect to be commercially available until at least 2024 and sales of U-235 that we do not expect to be commercially available for at least several years, if ever. As a result, we will need substantial additional financing to support our continuing operations and further the development of and commercialize our future isotopes.
58
Until such time as we can generate significant revenue from sales of our future isotopes, if ever, we expect to finance our cash needs through public or private equity or debt financings or other capital sources, including potential collaborations, licenses and other similar arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. Our ability to raise additional funds may be adversely impacted by potential worsening global economic conditions and the recent disruptions to, and volatility in, the credit and financial markets in the United States and worldwide resulting from the ongoing COVID-19 pandemic and otherwise. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise funds through collaborations, or other similar arrangements with third parties, we may have to relinquish valuable rights to our future isotopes, future revenue streams or research programs or may have to grant licenses on terms that may not be favorable to us and/or may reduce the value of our common stock. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market our future isotopes even if we would otherwise prefer to develop and market such isotopes ourselves.
Cash Flows
The following table sets forth a summary of our cash flows for the six-month period ended June 30, 2022 and the period from September 13, 2021 (inception) through December 31, 2021:
|
Six Months Ended |
For the |
|||||||
|
Cash used in operating activities |
$ |
(1,383,549 |
) |
$ |
(577,692 |
) |
||
|
Cash used in investing activities |
|
(1,732,595 |
) |
|
(2,988,210 |
) |
||
|
Cash provided by financing activities |
|
2,850,549 |
|
|
6,500,900 |
|
||
|
Net (decrease) increase in cash |
$ |
(265,595 |
) |
$ |
2,934,998 |
|
||
Operating Activities.
Net cash used in operating activities was $1,383,549 for the six months ended June 30, 2022 and was primarily due to our net loss of $1,684,758, adjusted for stock-based compensation expense of $231,548 and amortization of right-of-use asset of $37,476, partially offset by a $32,185 change in our operating assets and liabilities.
Net cash used in operating activities was $577,692 for the period from September 13, 2021 (inception) through December 31, 2021, and was primarily due to our net loss of $2,607,927, adjusted for stock-based compensation expense of $513,227, the issuance of warrants to purchase common stock of $1,735,841 and a $218,833 change in our operating assets and liabilities.
Investing activities.
Net cash used by investing activities was $1,732,595 and $2,988,210 for the six months ended June 30, 2022 and the period from September 13, 2021 (inception) through December 31, 2021, respectively, and was comprised of construction in progress.
Financing Activities.
Net cash provided by financing activities was $2,850,549 for the six months ended June 30, 2022 and was comprised primarily of net proceeds of $2,863,595 from the sale and issuance of 1,559,780 shares of our common stock and the repayment of notes payable of $13,046.
59
Net cash provided by financing activities was $6,500,900 for the period from September 13, 2021 (inception) through December 31, 2021 and was comprised primarily of net proceeds of $6,454,000 from the sale and issuance of 20,652,500 shares of our common stock in 2021.
Contractual Obligations and Commitments
We lease our research and development facility in Pretoria, South Africa under a lease with base monthly rent payment of approximately $8,000 with a term expiring on December 31, 2030.
As of June 30, 2022, we had commitments of approximately $7.8 million with Klydon for the ongoing development activities under the Turnkey Contract due within approximately 18 months.
In addition, we enter into contracts in the normal course of business with vendors for services and products for operating purposes. These contracts do not contain any minimum purchase commitments and generally provide for termination after a notice period, and, therefore, are not considered long-term contractual obligations. Payments due upon cancellation consist only of payments for services provided and expenses incurred up to the date of cancellation.
Off-Balance Sheet Arrangements
During the period presented we did not have, nor do we currently have, any off-balance sheet arrangements as defined under the rules and regulations of the SEC.
Critical Accounting Policies and Significant Judgments and Estimates
Our financial statements are prepared in accordance with generally accepted accounting principles in the United States. The preparation of our financial statements and related disclosures requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in our financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on a periodic basis. Our actual results may differ from these estimates.
While our significant accounting policies are described in more detail in the notes to our financial statements appearing elsewhere in this prospectus, we believe that the following accounting policies are critical to understanding our historical and future performance, as the policies relate to the more significant areas involving management’s judgments and estimates used in the preparation of our financial statements.
Research and Development Costs
As part of the process of preparing our financial statements, we are required to estimate our accrued research and development expenses as of each balance sheet date. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual costs. The majority of our service providers will invoice us in arrears for services performed, based on a pre-determined schedule or when contractual milestones are met, but some require advance payments. We make estimates of our accrued expenses as of each balance sheet date in the financial statements based on facts and circumstances known to us at that time. If timelines or contracts are modified based upon changes in the protocol or scope of work to be performed, we modify our estimates and accruals accordingly on a prospective basis.
We base our expenses related to external research and development services on our estimates of the services received and efforts expended pursuant to quotes and contracts with vendors that conduct research and development on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the expense. In accruing service fees, we estimate the
60
time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, we adjust the accrual or the amount of prepaid expenses accordingly.
Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in reporting amounts that are incorrect in any particular period.
Stock-Based Compensation
On October 3, 2021, our board of directors and stockholders approved the 2021 Plan. Under the 2021 Plan, stock-based awards are measured at fair value and recognized over the requisite service period. Forfeitures are accounted for in the period they occur. We estimate the fair value of each stock-based award on the date of grant using the Black-Scholes option pricing model which requires the input of subjective assumptions:
• Fair value of common stock. See the subsection entitled “— Determination of Fair Value of Common Stock” below.
• Risk-free interest rate. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of grant for zero coupon U.S. Treasury notes with maturities similar to the expected term of the awards.
• Expected dividend yield. We base the expected dividend yield assumption on the fact that we have never paid cash dividends and have no present intention to pay cash dividends and, therefore, used an expected dividend yield of zero.
• Expected volatility. Since we are not yet a public company and do not have a trading history for our common stock, the expected volatility assumption is based on volatilities of a peer group of similar companies whose share prices are publicly available. The peer group was developed based on companies in the biotechnology industry. We will continue to apply this process until a sufficient amount of historical information regarding the volatility of our own stock price becomes available.
• Expected life. The expected life represents the period of time that options are expected to be outstanding. Because we do not have historical exercise behavior, we determine the expected life assumption using the simplified method, for employees, which is an average of the contractual term of the option and its vesting period. The expected term for nonemployee options is equal to the contractual term.
The fair value of our awards for the six months ended June 30, 2022 has been estimated using Black-Scholes based on the following assumptions: term of 5.5 to 5.8 years; volatility of 63.0% to 64.5%; risk-free rate of 1.68% – 3.08%; and no expectation of dividends. The fair value of our awards as of December 31, 2021 has been estimated using Black-Scholes based on the following assumptions: term of 5.77 years; volatility of 69.5%; risk-free rate of 1.11%; and no expectation of dividends.
As of June 30, 2022, the unrecognized stock-based compensation expense related to employee stock options was $2,303,866 and is expected to be recognized as expense over a weighted-average period of approximately 2.4 years. The intrinsic value of all outstanding stock options as of June 30, 2022 was approximately $262,500, based on the fair value price of $2.00 per share, of which approximately $58,333 related to exercisable options and approximately $204,167 related to unexercisable options.
Determination of Fair Value of Common Stock
As there has been no public market for our common stock to date, the estimated fair value of our common stock has been determined by our board of directors as of the date of each option grant, with input from management, and recent third-party financings consummated by the Company.
61
Our board of directors considered various objective and subjective factors to determine the fair value of our common stock as of each grant date, including:
• our stage of development and material risks related to our business;
• the progress of our research and development programs;
• our business conditions and projections;
• our financial position and our historical and forecasted performance and operating results;
• the lack of an active public market for our common stock;
• the prices of our common stock sold to or exchanged between outside investors in arm’s length transactions;
• the analysis of initial public offerings and the market performance of similar companies in the isotopes industry;
• the likelihood of achieving a liquidity event, such as an initial public offering or sale of our company in light of prevailing market conditions;
• the hiring of key personnel and the experience of management;
• trends and developments in the isotopes industry; and
• external market conditions and trends affecting the isotopes industry.
Following the closing of this offering, our board of directors will determine the fair market value of our common stock based on its closing price as reported on the date of grant on the primary stock exchange on which our common stock is traded.
Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk
As of June 30, 2022 and December 31, 2021, our cash consists of cash in readily available checking accounts. We do not hold any short-term investments. As a result, the fair value of our portfolio is relatively insensitive to interest rate changes. As of June 30, 2022 and December 31, 2021, we had no bank debt outstanding and are therefore not exposed to interest rate risk with respect to debt. We believe a hypothetical 100 basis point increase or decrease in interest rates during the period presented would not have had a material impact on our financial results.
Foreign Currency Risk
Our expenses are generally denominated in U.S. dollars but our operations are currently primarily located outside the United States and we have entered into a number of contracts with vendors that are denominated in foreign currencies. We are subject to foreign currency transaction gains or losses on our contracts denominated in foreign currencies. To date, foreign currency transaction gains and losses have not been material to our financial statements, and we have not had a formal hedging program with respect to foreign currency. We believe a hypothetical 100 basis point increase or decrease in exchange rates during the period presented would not have had a material impact on our financial results.
Effects of Inflation
Inflation generally affects us by increasing our cost of labor and research and development costs. We do not believe that inflation and changing prices had a significant impact on our results of operations for the period presented herein.
62
Emerging Growth Company and Smaller Reporting Company Status
We are an “emerging growth company” under the JOBS Act, and as such, we can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to delay the adoption of accounting standards that have different effective dates for public and private companies until those standards would otherwise apply to private companies. We have elected to avail ourselves of this exemption from new or revised accounting standards, and therefore we will not be subject to the same requirements to adopt new or revised accounting standards as other public companies that are not emerging growth companies.
We will cease to be an emerging growth company prior to the end of such five-year period if certain earlier events occur, including if we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, our annual gross revenues exceed $1.07 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period. In particular, in this prospectus, we have not included all of the executive compensation related information that would be required if we were not an emerging growth company, and we may elect to take advantage of other reduced reporting requirements in future filings.
We are also a “smaller reporting company” as defined in the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as our voting and non-voting common stock held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter, or our annual revenue is less than $100.0 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
63
Overview
We are a pre-commercial stage advanced materials company dedicated to the development of technology and processes that, if successful, will allow for the enrichment of natural isotopes into higher concentration products, which could be used in several industries. We have an exclusive license to use proprietary technology, the Aerodynamic Separation Process (“ASP technology”), originally developed and licensed to us by Klydon Proprietary Ltd (“Klydon”), for the production, distribution, marketing and sale of all isotopes. Our initial focus is on the production and commercialization of enriched of Molybdenum-100 (“Mo-100”). Klydon has agreed to provide us a first commercial-scale Mo-100 enrichment plant located in South Africa with a manufacturing capacity of 20 kg/year of 95% enriched Mo-100 when fully operational. We believe that the Mo-100 we may develop using the ASP technology has significant potential advantages for use in the preparation of nuclear imaging agents by radiopharmacies and others in the medical industry. We also intend to use the ASP technology to produce enriched Uranium-235 (“U-235”). We believe that the U-235 we may develop using the ASP technology may be commercialized as a nuclear fuel component for use in the new generation of HALEU-fueled small modular reactors that are now under development for commercial and government uses.
We may also seek to use the ASP technology to separate Silicon-28, which we believe has potential application in the quantum computing target end market, and Carbon-14, which we believe has potential application in the pharma/agrochem target end market. In addition, we are considering future development of the ASP technology for the separation of Zinc-68, Ytterbium-176, Zinc-67, Nickel-64 and Xenon-136 for potential use in the healthcare target end market, and Chlorine -37 and Lithium-6 for potential use in the nuclear energy target end market.
The aerodynamic separation technique has its origins in the South African uranium enrichment program in the 1980s and the ASP technology has been developed during the last 18 years by the scientists at Klydon. In Klydon’s testing, the ASP technology has demonstrated efficacy and commercial scalability in the enrichment of oxygen-18 and silicon-28. ASP Isotopes Inc. was incorporated in Delaware in September 2021 to acquire assets and license intellectual property rights related to the production of Mo-100 using the ASP technology. In January 2022 we also licensed intellectual property rights related to the production of U-235 using the ASP technology.
We operate principally through subsidiaries: ASP Isotopes Guernsey Limited (the holding company of ASP Isotopes South Africa (Proprietary) Limited), which will be focused on the development and commercialization of high value, low volume isotopes for highly specialized end markets (such as Mo-100 and others, including Silicon-28); Enriched Energy LLC, which will be focused on the development and commercialization of uranium for the nuclear energy market; and ASP Isotopes UK Ltd, which is the licensee of the ASP technology under the exclusive license agreement with Klydon.
Our Strategy
Complete development and commissioning of Mo-100 enrichment facility located in Pretoria, South Africa.
We intend to complete the development and construction of our first Mo-100 enrichment facility located in Pretoria, South Africa. In October 2021, we acquired physical assets, including equipment, of Molybdos (Pty) Limited (Molybdos) located at the plant after having been declared the winner of a competitive auction process under Section 45 of the South Africa Consumer Protection Act, 2008 (the Molybdos Business Rescue Auction), and we licensed the ASP technology for the production of Mo-100 from Klydon Proprietary Ltd (Klydon). We subsequently entered into a turnkey contract with Klydon pursuant to which Klydon has agreed to provide us a first commercial-scale Mo-100 enrichment plant. The activities to be undertaken or performed by Klydon include: taking control of the assets acquired by us in the Molybdos Business Rescue Auction; the design of a Mo-100 enrichment facility with a manufacturing capacity of 20 kg/year of 95% enriched Mo-100 when fully operational; the supply of required components, equipment and labor; the installation, testing and commissioning of the Mo-100 enrichment facility, including production of targets to be used by customers in cyclotrons; securing all required approvals, regulatory authorizations and other required consents for the operation of the plant; providing training to local ASP Isotopes South Africa (Proprietary) Limited personnel to enable them to operate the plant going forward; and providing warranties in relation to the performance targets of the plant which are required to be met. Klydon will also be responsible for liaising with the relevant South African authorities including the South African Non
64
Proliferation Council, the Nuclear Suppliers Group and International Atomic Energy Agency to ensure that the Mo-100 enrichment plant is compliant with international laws and guidelines. We initiated Phase 1 of the Mo-100 development plan under the turnkey contract, targeting 5 kg/year of 95% enriched Mo-100, in October 2021. While we expect to complete this stage in the second half of 2022, it is possible that it may take longer than anticipated to complete due to unexpected delays. Upon completion of Phase 1, we expect to initiate Phase 2, which targets expanded production of up to 20 kg/year of 95% enriched Mo-100. After the commissioning process, which could take 12 months, we expect to commence commercialization of Mo-100 in 2024. While Klydon is responsible for the construction of the enrichment facility, we are responsible for the commercial operation of the enrichment facility, and we expect to recruit the required workforce during 2022.
Demonstrate the capability to produce Mo-100 using the ASP technology and capitalize on the opportunity to solve the Mo-99 supply chain challenges in the existing medical isotope market.
We intend to demonstrate the capability to produce Mo-100 at a scale that can support anticipated customer demand for Mo-100 as alternative and potentially more convenient production route for Tc-99m used in nuclear medical diagnostic procedures. Mo-99’s decay product, technetium-99m (Tc-99m), is used in medical procedures to diagnose heart disease and cancer, to study organ structure and function, and to perform other important medical applications. We intend to offer our Mo-100 to customers that may convert Mo-100 into Mo-99 or directly into Tc-99m, and we believe that the use of Mo-100 in this way will be an attractive alternative route to production of Tc-99m for a number of reasons.
• Only a small number of major reactors located around the world (e.g., Australia, Belgium, the Netherlands and South Africa) produce large-scale amounts of Mo-99, and these reactors are taken off-line periodically for refueling and maintenance and go off-line on an unscheduled basis due to the need for extended repairs, which results in a global Mo-99 supply chain that is lengthy, complex and prone to interruption and has experienced supply shortages. Customers that could use and stockpile Mo-100 would not have to manage the periodic shortages and supply chain challenges related to Mo-99.
• Mo-99 (a radioisotope with a 66-hour half-life) decays and loses activity in transit, so it must be moved through the supply chain quickly to minimize decay losses and it cannot be stockpiled. Mo-100 (a stable isotope of molybdenum that does not decay) will not decay in transit, so the supply chain would not be dependent on elapsed time from production of Mo-100 to the delivery of a Tc-99m dose to a hospital or clinic.
• Mo-99 (with decay product Tc-99m) must be shipped in shielded transport containers that comply with the regulatory requirements for safe transport of radioactive material. Mo-100 is stable (non-radioactive) and therefore does not have the same handling and shipping requirements.
Mo-100 produced using ASP technology could support an alternative and potentially more convenient production route for Tc-99m.
We believe that Mo-100 that we may produce using the ASP technology is well-positioned to provide an alternative route to producing Tc-99m. Customers will be able to convert Mo-100 into Mo-99 using a cyclotron or a linear accelerator. The Mo-99 can then be converted into Tc-99m using a technetium generator, which is currently the standard method used by hospitals and healthcare providers to obtain their Tc-99m. It is highly likely that the technetium generator used in this process will require some modifications, relative to the technetium generators currently available. These modified technetium generators will require approval from healthcare regulators. Currently, three potential customers are in the advanced stages of this regulatory approval process for a modified generator with relevant healthcare regulators. Customers could also convert Mo-100 directly into Tc-99m using a cyclotron, which would eliminate the need for a technetium generator. To date, only one healthcare regulator (Health Canada) has approved the use of Tc-99m that has been directly produced from Mo-100 in a low powered cyclotron. We believe it is likely that healthcare regulators in other countries will also require clinical data to support the use of Tc-99m that is produced directly from Mo-100.
Continue identifying potential offtake customers and strategic partners for Mo-100.
We have already seen significant interest from potential offtake customers for Mo-100 that we may produce using the ASP technology. We have had or are currently in active dialogue or entered into non-binding LOIs with six potential offtake customers that could use the entire anticipated annual capacity of the initial plant. In addition, to support our efforts in identifying potential customers in China, we have entered into a consulting and sales
65
commission agreement with a strategic advisor. We expect to enter into similar consulting and sales commission agreements with other individuals and entities in the future in the ordinary course of business. Globally, there are potentially hundreds of potential offtake customers with varying demands from a few grams to kilogram quantities. We plan to continue identifying new potential offtake customers, with the assistance of strategic advisors where helpful, for our Mo-100 with a view to securing additional volume for future Mo-100 enrichment plants.
Demonstrate the capability to produce high-assay low-enriched uranium (HALEU) using the ASP technology and meet anticipated demand for the new generation of HALEU-fueled small modular reactors and advanced reactor designs that are now under development for commercial and government uses.
We plan to begin the enrichment of uranium to demonstrate our capability to produce HALEU using the ASP technology. We anticipate a future demand for HALEU for the new generation of HALEU-fueled small modular reactors (SMRs) and advanced reactor designs that are now under development for commercial and government uses. SMRs are viewed as being cheaper, safer, and more versatile than traditional large scale nuclear reactors and development of the new technology is receiving considerable funding from the U.S. Department of Energy, as well as from the governments of other countries. There is currently no commercial production of HALEU in the United States and there has been a reliance on delivery from other countries, particularly Russia. We are currently conducting a feasibility study with respect to constructing an enrichment facility in either the United States or an international location. We would need to obtain approval of the U.S. Nuclear Regulatory Commission to produce HALEU in a U.S.-based facility.
Our Strengths
ASP technology developed by Klydon.
The aerodynamic separation technique has its origins in the South African uranium enrichment program in the 1980s and the ASP technology has been developed during the last 18 years by the scientists at Klydon. To date, the scientists at Klydon have constructed two ASP plants for the enrichment of oxygen-18 and silicon-28 in Pretoria, South Africa, which were commissioned in October 2015 and July 2018, respectively, and remain fully operational. While the technology has not yet been used to enrich either Molybdenum or Uranium, we believe the success of the enrichment process for oxygen-18 and silicon-28 has demonstrated the efficacy and commercial scalability of the ASP technology from laboratory to commercial. We have exclusive worldwide licenses from Klydon for the production of Mo-100 and U-235 and, if our research and development is successful (and subject to obtaining applicable regulatory approvals and appropriate licenses), we plan to commercialize Mo-100 and U-235 produced using the ASP technology. To date, we have not built a functioning Mo-100 or U-235 enrichment plant or even demonstrated the ability to produce Mo-100 or U-235 using the ASP technology.
High barriers to entry.
We have exclusive worldwide licenses to the ASP technology for the production of Mo-100 and U-235. Klydon has spent the last 18 years and tens of millions of dollars developing the aerodynamic separation technique used in the ASP technology, generating critical trade secrets. We believe our competitors lag behind us in terms of the technical expertise of our senior management and the know-how contained in the aerodynamic separation technique, and will be unable to replicate the expected results of the ASP technology, even as we expect to continue to improve the existing technology and processes. Additionally, the high capital costs of development of proprietary technologies, significant lead times required to construct new enrichment facilities, as well as stringent regulatory and operating requirements applicable to enrichment facilities, adds to the significant barriers to entry for smaller competing market participants.
ASP technology is a flexible platform with the potential to produce HALEU that could serve a large addressable market.
ASP technology is a flexible platform, compact in size and weight, and easily scaled to industrial level with number of separation devices added in parallel. The ASP technology also has few moving parts, with low capital and operating costs in comparison to alternatives. We believe that, assuming receipt of required regulatory approvals and governmental permits, the ASP technology can be deployed quickly and with a relatively minimal capital cost, which offers better speed-to-market dynamics for HALEU produced using ASP technology than our competitors. We believe that the ASP technology is well-positioned to address a substantial global HALEU market that is contemplating a transition from petroleum-based energy to energy produced in a new generation of HALEU-fueled SMRs and advanced reactors.
66
ASP technology is designed to be low cost, low energy, and environmentally friendly.
We are completing the development of our first Mo-100 enrichment facility utilizing the ASP technology located in Pretoria, South Africa. The ASP technology is designed to be scalable, low cost, low energy, and environmentally friendly, with no radioactive waste or hazardous materials produced in the process and planned arrangements to reuse chemical byproducts. The enrichment plant is initially targeting 5 kg/year of 95% enriched Mo-100 and then expanded production of up to 20 kg/year of 95% enriched Mo-100. If successful, we believe we will have the opportunity to offer customers an alternative and potentially more convenient production route for Tc-99m used in nuclear medical diagnostic procedures. If successful, we believe we will have opportunities to shorten the existing supply chain and reduce international transportation of radioactive material for nuclear medical diagnostic procedures.
Experienced team
Our board of directors and advisers have specialized expertise in isotopes enrichment, R&D, technology, plant development and manufacturing. Dr Einar Ronander, who serves as Chief Scientific Adviser to our board of directors, and Dr Hendrik Strydom, one of our directors, previously co-founded and currently serve as Executive Chairperson and Chief Executive Officer, respectively, of Klydon. Prior to founding Klydon, Dr Ronander and Dr Strydom worked at the South African Atomic Energy Corporation (AEC) focusing on uranium laser enrichment. The scientific team at Klydon combined has decades of experience in research and development of isotopes enrichment and amassed deep knowledge in the field. The Klydon team’s knowledge of isotopes enrichment underpins our research and discovery efforts under the turnkey contract with Klydon.
Our board of directors and our management team also has broad experience and successful track records in biopharmaceutical research, chemicals, manufacturing and commercialization, as well as in business, operations, and finance. Our board of directors’ and management team’s experience was gained at leading companies and financial institutions that include, Barclays Capital, Coty Inc, Deutsche Bank, Highbridge Capital, La Perla Beauty Ltd, Lehman Brothers, LyondellBasell and Morgan Stanley.
Technical Background
What are Isotopes?
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties.
The number of protons within the atom’s nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom’s mass number, and each isotope of a given element has a different mass number. For example, carbon-12, carbon-13, and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13, and 14, respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons so that the neutron numbers of these isotopes are 6, 7, and 8 respectively.
There are 23 isotopes of Silicon, all of which have 14 protons and 14 neutrons but have between 8 and 30 neutrons. The table below shows a selection of those isotopes. Three isotopes are stable which have mass numbers of 28, 29 and 30 which have 14, 15 and 16 neutrons respectively. The other 20 isotopes are radioactive and decay with short half lives and are therefore do not typically exist in naturally occurring silicon. In naturally occurring silicon, the isotope with atomic mass of 28 is usually the most abundant, typically accounting for approximately 92.22% of the material. The isotope with atomic mass of 29 typically accounts for 4.69% of the material and the isotope with atomic mass of 30 typically accounts for 3.09% of the material.
Molybdenum has 33 known isotopes, ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. All unstable isotopes of molybdenum decay into isotopes of zirconium, niobium, technetium, and ruthenium.
67
Uranium is a naturally occurring radioactive element that has no stable isotope. It has two primordial isotopes, uranium-238 and uranium-235, which have long half-lives and are found in appreciable quantity in the Earth’s crust. The decay product, uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from U-214 to U-242 (with the exception of U-220 and U-241). The standard atomic weight of natural uranium is 238.02891 with 99.27% of naturally occurring uranium being the isotope with an atomic mass of 238.051.
|
Selected isotopes of Silicon |
Selected isotopes of Molybdenum |
Selected isotopes of Uranium |
||||||||||||||||||||||||||||||||
|
Nuclide |
Protons |
Neutrons |
Isotopic Mass |
Half |
Natural abundance |
Nuclide |
Protons |
Neutrons |
Isotopic Mass |
Half |
Natural abundance |
Nuclide |
Protons |
Neutrons |
Isotoopic |
Half |
Natural |
|||||||||||||||||
|
22 |
14 |
8 |
22.036 |
29 ms |
91 |
42 |
49 |
90.912 |
15.49 min |
225 |
92 |
133 |
225.029 |
62 ms |
||||||||||||||||||||
|
23 |
14 |
9 |
23.025 |
42.3 ms |
92 |
42 |
50 |
91.907 |
Stable |
14.65% |
226 |
92 |
134 |
226.029 |
269 ms |
|||||||||||||||||||
|
24 |
14 |
10 |
24.012 |
140 ms |
93 |
42 |
51 |
92.907 |
4000 y |
227 |
92 |
135 |
227.031 |
1.1 m |
||||||||||||||||||||
|
25 |
14 |
11 |
25.004 |
220 ms |
94 |
42 |
52 |
93.905 |
Stable |
9.19% |
228 |
92 |
136 |
228.031 |
9.1 m |
|||||||||||||||||||
|
26 |
14 |
12 |
25.992 |
2.245 s |
95 |
42 |
53 |
94.906 |
Stable |
15.87% |
229 |
92 |
137 |
229.034 |
57.8 m |
|||||||||||||||||||
|
27 |
14 |
13 |
26.987 |
4.15 s |
96 |
42 |
54 |
95.905 |
Stable |
16.67% |
230 |
92 |
138 |
230.034 |
20.23 d |
|||||||||||||||||||
|
28 |
14 |
14 |
27.977 |
Stable |
92.22% |
97 |
42 |
55 |
96.906 |
Stable |
9.58% |
231 |
92 |
139 |
231.036 |
4.2 d |
||||||||||||||||||
|
29 |
14 |
15 |
28.977 |
Stable |
4.69% |
98 |
42 |
56 |
97.905 |
Stable |
24.29% |
232 |
92 |
140 |
232.037 |
68.9 y |
||||||||||||||||||
|
30 |
14 |
16 |
29.974 |
Stable |
3.09% |
99 |
42 |
57 |
98.908 |
2.75 d |
233 |
92 |
141 |
233.04 |
1.592 e5 y |
Trace |
||||||||||||||||||
|
31 |
14 |
17 |
30.975 |
157.36 min |
100 |
42 |
58 |
99.907 |
Stable |
9.74% |
234 |
92 |
142 |
234.041 |
2.455 e5 y |
Trace |
||||||||||||||||||
|
32 |
14 |
18 |
31.974 |
153 y |
trace |
101 |
42 |
59 |
100.910 |
14.61 m |
235 |
92 |
143 |
235.044 |
7.038 e8 y |
0.72% |
||||||||||||||||||
|
33 |
14 |
19 |
32.978 |
6.18 s |
102 |
42 |
60 |
101.910 |
11.3 m |
236 |
92 |
144 |
236.046 |
2.342 e7 y |
Trace |
|||||||||||||||||||
|
34 |
14 |
20 |
33.979 |
2.77 s |
103 |
42 |
61 |
102.913 |
67.5 s |
237 |
92 |
145 |
237.049 |
6.752 d |
Trace |
|||||||||||||||||||
|
35 |
14 |
21 |
34.985 |
780 ms |
104 |
42 |
62 |
103.914 |
60 s |
238 |
92 |
146 |
238.051 |
4.468 e9 y |
99.27% |
|||||||||||||||||||
|
36 |
14 |
22 |
35.987 |
450 ms |
105 |
42 |
63 |
104.917 |
35.6 s |
239 |
92 |
147 |
239.054 |
23.45 m |
||||||||||||||||||||
|
37 |
14 |
23 |
36.993 |
90 ms |
106 |
42 |
64 |
105.918 |
8.73 s |
240 |
92 |
148 |
240.057 |
14.1 h |
Trace |
|||||||||||||||||||
|
38 |
14 |
24 |
37.996 |
90 ms |
107 |
42 |
65 |
106.922 |
3.5 s |
242 |
92 |
150 |
242.063 |
16.8 m |
||||||||||||||||||||
Methods of Separation and Enrichment of Isotopes
Isotope enrichment is the process of concentrating specific isotopes of a chemical element by removing other isotopes. During the last century, a number of different methods have been developed to separate and enrich isotopes. The current separation or enrichment processes are based either on the atomic weight of the isotope, small differences in chemical reaction rates produced by different atomic weights or are based on properties not directly connected to atomic weight such as nuclear resonances.
Diffusion
Often performed on gases, but also on liquids, the diffusion method relies on the fact that in thermal equilibrium, two isotopes with the same energy will have different average velocities. The lighter atoms (or the molecules containing them) will travel more quickly and be more likely to diffuse through a membrane. The difference in speeds is proportional to the square root of the mass ratio, so the amount of separation is small and many cascaded stages are needed to obtain high purity. This method is expensive due to the work needed to push gas through a membrane and the many stages necessary.
Centrifugal
Centrifugal methods rapidly rotate the material allowing the heavier isotopes to go closer to an outer radial wall. This too is often done in gaseous form using a Zippe-type centrifuge.
A Zippe-type centrifuge relies on the force resulting from centripetal acceleration to separate molecules according to their mass, and can be applied to most fluids. The dense (heavier) molecules move towards the wall and the lighter ones remain close to the center. The centrifuge consists of a rigid body rotor rotating at full period at high speed. Concentric gas tubes located on the axis of the rotor are used to introduce feed gas into the rotor and extract the heavier and lighter separated streams. For U-235 production, the heavier stream is the waste stream and the lighter stream is the product stream. Modern Zippe-type centrifuges are tall cylinders spinning on a vertical axis, with a vertical temperature gradient applied to create a convective circulation rising in the center and descending at the periphery of the centrifuge. Diffusion between these opposing flows increases the separation by the principle of countercurrent multiplication.
68
In practice, since there are limits to how tall a single centrifuge can be made, several such centrifuges are connected in series. Each centrifuge receives one input and produces two output lines, corresponding to light and heavy fractions. The input of each centrifuge is the output (light) of the previous centrifuge and the output (heavy) of the following stage. This produces an almost pure light fraction from the output (light) of the last centrifuge and an almost pure heavy fraction from the output (heavy) of the first centrifuge.
Electromagnetic
Electromagnetic separation is mass spectrometry on a large scale, so it is sometimes referred to as mass spectrometry. It uses the fact that charged particles are deflected in a magnetic field and the amount of deflection depends upon the particle’s mass. It is very expensive for the quantity produced, as it has an extremely low throughput, but it can allow very high purities to be achieved. This method is often used for processing small amounts of pure isotopes for research or specific use (such as isotopic tracers), but is impractical for industrial use.
Laser
In this method, a laser is tuned to a wavelength which excites only one isotope of the material and ionizes those atoms preferentially. The resonant absorption of light for an isotope is dependent upon its mass and certain hyperfine interactions between electrons and the nucleus, allowing finely tuned lasers to interact with only one isotope. After the atom is ionized it can be removed from the sample by applying an electric field. This method is often abbreviated as AVLIS (atomic vapor laser isotope separation). This method has only recently been developed as laser technology has improved, and is currently not used extensively.
Chemical Methods
Although isotopes of a single element are normally described as having the same chemical properties, this is not strictly true. In particular, reaction rates are very slightly affected by atomic mass. Techniques using this are most effective for light atoms such as hydrogen. Lighter isotopes tend to react or evaporate more quickly than heavy isotopes, allowing them to be separated. This is how heavy water is produced commercially
Gravity
Isotopes of carbon, oxygen, and nitrogen can be purified by chilling these gases or compounds nearly to their liquefaction temperature in very tall (200 to 700 feet (61 to 213 m)) columns. The heavier isotopes sink and the lighter isotopes rise, where they are easily collected.
The Aerodynamic Separation Process (ASP) Technology
ASP technology is proprietary technology licensed from Klydon which succeeds earlier work, first detailed in the scientific media in the mid-1970s, relating to an industrial scale enrichment plant for uranium that was constructed utilizing the so-called “stationary-wall centrifuge”. The original technology was highly energy consuming and was not able to compete on an economic basis with other methods of isotope separation. The innovative development of the ASP technology over the past 18 years has culminated in a more advanced separation device that we believe can compete on a commercial scale with other methods of isotope separation. The ASP separation device separates both gas species and isotopes in a volatile state via an approximate flow pattern as shown below.
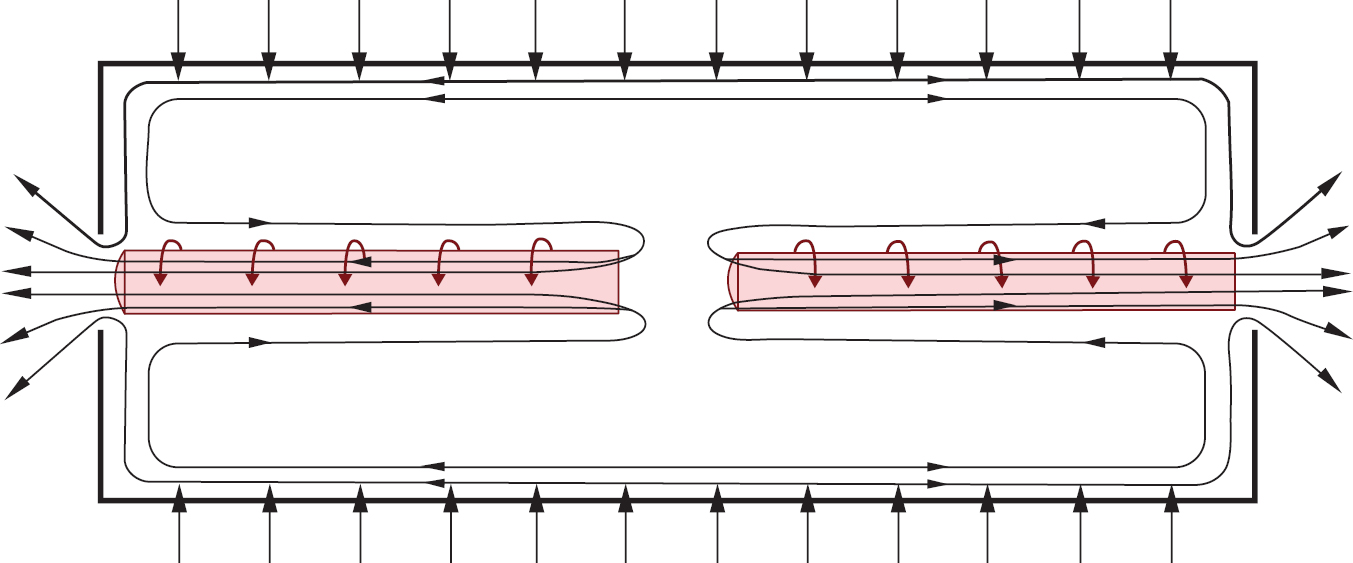
Gas flow pattern inside ASP separation device.
69
The ASP enrichment process uses an aerodynamic technique similar to a stationary wall centrifuge. The isotope material in raw gas form enters the stationary tube at high speed by tangential injection through finely placed and sized openings in the surface of the tube. The gas then follows a flow pattern that results in two gas vortexes occurring around the geometrical axis of the separator. The isotope material becomes separated in the radial dimension as a result of the spin speed of the isotope material reaching several hundred meters per second. An axial mass flow component in each tube feeds isotope material to the respective ends of the separator where the collection of the portions of isotope material is accomplished.
The advantages of ASP technology are as follows:
• No moving parts, with low capital and operating costs in comparison to alternatives.
• Compact in size and weight.
• Easily scaled to industrial level with number of separation devices added in parallel.
• The separation process occurs inside a closed cylindrical container and is a volume technology, i.e., the process efficiency is not affected by poisoning of surface contaminates as is the case for surface separation processes.
• ASP operates very efficiently at molecular masses below 100 atomic mass units, unlike other separation processes which are more efficient higher masses, which ASP can achieve equally well or to a superior degree.
• ASP easily separates hydrogen gas from other gas components, e.g., harvesting hydrogen gas from carbon monoxide and carbon dioxide and altering the ratio of syngas mixture.
• With the right material choice ASP handles even the most corrosive gases.
• ASP can separate any isotopes that have a gaseous or volatile chemical compound.
• Most of the subsystems are procured from off-the-shelf components.
• An ASP plant can be constructed in any country that adheres to the International Atomic Energy Agency (IAEA) protocols for the protection of dual use technology.
ASP Plant Configuration
The figure below shows a schematic of an ASP cascade in operation. The cascade consists of several enrichment stages, connected in a 1-up-1-down cascade configuration. The stages can be grouped into segments. (This method of organizing stages is not reflected in the figure)
70
The bold blue arrows represent flows of the element into and out of the cascade:
• H is the product, enriched in the isotope
• L is the tails, stripped of the isotope
• F = FX + FY is the feed stream at natural isotopic composition:
• FX is the feed into the product stream of an adjoining stage.
• FY is the feed into the tails stream of an adjoin
Each stage in the cascade is operated in one of two configurations:
(1) A net backward flow of the isotope: Xi > Yi. These stages are referred to as “product”, situated in the so-called “product cascade section”, and their flows are marked with an “H” subscript.
(2) A net forward flow of isotope: Xi < Yi. These stages are referred to as “tails”, situated in the so-called “tails cascade section”, and their flows are marked with an “L” subscript.
The red arrows represent the addition or extraction of carrier gas from the process. The arrows have been added for clarity and orientation, but the mass flows of the carrier gas will be ignored in the rest of the discussion as it pertains to the isotope mass flows only (as represented by the blue arrows). The carrier gas mass flows can be superimposed on any isotope mass balance using the molar mass characteristics of the ASP stages (see below).
The block marked “GS” represents the gas separator: a piece of equipment used to separate the carrier gas from the element of interest to the degree necessary to provide a suitable reflux stream to the tails cascade section.
The blue squares are simply suitable areas where streams can be split or mixed.
An ASP stage is characterized by functions of Y, the flow of isotope in its tails stream. The characteristics of interest are:
• α(Y): the separation factor between the tails and product streams.
• MY(Y): the molar mass of the tails stream.
• MX(Y): the molar mass of the product stream.
• P(Y): the stage’s power usage.
• X(θ,Y): the flow of Zinc in the product stream, where θ = Y/(X+Y) is the cut defined in terms of isotope flows.
Note the following:
• α is the ratio of the tails and product stream abundance ratios.
• Y, X(θ,Y) and α(Y) describe the stage’s behaviour with regards to Zinc, while MY(Y) and MX(Y) defines its behaviour with regards to the carrier gas.
• P, the stage’s power usage, depends on the ASP separator, but also on factors such as compressor efficiency, friction losses etc. It is therefore a partial function of stage design.
• It is possible to define Pmin, the theoretical minimum energy usage of a stage, by assuming 100% efficient compressors and no losses in the stage. Pmin is a function of the ASP separator only. In practice P is a more useful metric, as the contribution of compressor inefficiencies to power consumption is significant.
• Except for X, the stage’s characteristics are not defined in terms of the cut θ, as they are simply not sensitive to it above a certain lower limit θmin. In practice θmin is small enough that it has no influence on the normal operating envelope of the stage.
• X is per definition a function of Y via θ as indicated.
71
The cut of an ASP stage can be dynamically adjusted to any value larger than θmin, allowing its operating point to be changed online during production.
All stages in the product cascade section are operated at the same point < XH,YH >, where XH > YH, ensuring that a net backward flow of the process element, H = XH — YH is achieved. This corresponds to a cut of less than 50% and ensures a positive flow of enriched product.
All stages in the tails cascade section are operated at the same point < XL,YL >, where XL < YL, ensuring that a net backward flow of the process element, L = XL — YL is achieved. This corresponds to a cut of more than 50% and ensures a positive flow of stripped tails.
Depending on the production requirements of the cascade the product and tails section operation points can be moved relative to each other during production, obtaining different combinations of H and L (and therefore different feeds F = H + L). The smaller H (or L) is chosen, the closer the product (or tails) section cut moves to 50%. If all stages are operated at a cut of 50%, the cascade is operated at full reflux, no product, tails, or feed streams are present, and the maximum process element concentration gradient will exist.
ASP Technology In Use
To date, the scientists at Klydon have constructed two ASP plants for the enrichment of oxygen-18 and silicon-28 in Pretoria, South Africa, which were commissioned in October 2015 and July 2018, respectively, and remain fully operational. We believe the success of the enrichment of oxygen-18 and silicon-28 has demonstrated the efficacy and commercial scalability of the ASP technology. We are currently constructing a Mo-100 enrichment plant, which, if successful, will produce Mo-100 for use in the preparation of nuclear imaging agents by radiopharmacies and others in the medical industry.
Nuclear Medicine
Nuclear medicine is a medical specialty that utilizes radioactive isotopes, referred to as radionuclides, to diagnose and treat disease. These radionuclides are incorporated into radiopharmaceuticals and introduced into the body by injection, swallowing, or inhalation. Physiologic/metabolic processes in the body concentrate the tracers in specific tissues and organs; the radioactive emissions from the tracers can be used to noninvasively image these processes or kill cells in regions where radionuclides have concentrated.
Other types of noninvasive diagnostic procedures — for example, computed tomography (CT) and magnetic resonance imaging (MRI) — can detect anatomical changes in tissues and organs as the result of disease. Nuclear medicine procedures can often detect the physiological and metabolic changes associated with disease before any anatomical changes occur. Such procedures can be used to identify disease at early stages and evaluate patients’ early responses to therapeutic interventions.
Single Photon Emission Computed Tomography (SPECT) generates three-dimensional (3D) images of tissues and organs using radionuclides that emit gamma rays; the most used radionuclide is Technitium-99m (Tc-99m), often referred to as the ‘work-horse’ of nuclear medicine. Individual gamma rays emitted from the decay of these radionuclides (i.e., single photon emissions) are detected using a gamma camera. This camera technology is used to obtain two-dimensional (2D) images; 3D SPECT images are computer generated from many 2D images recorded at different angles.
Positron Emission Tomography (PET) generates 3D images of tissues and organs using tracers that emit positrons (i.e., positive electrons): for example, fluorine-18 (F-18). Annihilation reactions between the positrons from these radionuclides and electrons present in tissues and organs produce photons. (Two photons are emitted simultaneously for each annihilation reaction and essentially travel in opposite directions.) The photon pairs are detected with a camera having a ring of very fast detectors and electronics. PET images generally have a higher contrast and spatial resolution than do SPECT images. However, PET equipment is more expensive and therefore not as widely available as SPECT equipment. Additionally, most PET tracers have short half-lives (e.g., nitrogen-13 (N-13): 10 minutes, carbon-11 (C-11): 20 minutes, and F-18: 110 minutes), so they must be produced close to their point of use.
72
Technetium-99m (Tc-99m)–the most widely used radioisotope in Nuclear Imaging
Tc-99m is used in approximately 80 percent of all nuclear medicine procedures performed worldwide each year.
Tc-99m is a particularly useful imaging radionuclide because it:
• Has a sufficiently long half-life (~6 hours) to be usable in nuclear medicine procedures.
• Emits energetic gamma rays (140 kiloelectron volts [keV]) that can be detected efficiently with widely available camera technologies.
• Provides low patient doses for some procedures because of its short half-life and lack of alpha or beta radiations
Tc-99m-based radiopharmaceuticals are used to diagnose disease in many tissue and organ systems, including bone, brain, heart, kidneys, liver, and lungs. About 50 percent of Tc-99m utilization in the United States is in nuclear cardiology, predominantly for myocardial perfusion imaging which images blood flow through heart muscle.
Because Tc-99m has a half life of just 6 hours, it cannot be stored or shipped long distances and it is currently produced using a technetium generator, which contains Molybdenum-99 which has a half-life of about 66-hours. In the reactor, Mo-99 decays to Tc-99m by emitting a beta particle (an electron). About 88 percent of the decays produce Tc-99m, which subsequently decays to the ground state, Tc-99g, by emitting a gamma ray. About 12 percent of the decays produce Tc-99g directly. Tc-99g decays to stable (i.e., nonradioactive) ruthenium-99 (Ru-99) after emitting a beta particle.
Technetium generators are systems that store Mo-99 and allow its decay product, Tc-99m, to be recovered for use. Most technetium generators are designed to be used with high-specific-activity Mo-99 (>1,000 Ci/g) produced by U-235 fission. The generator consists of an alumina (Al2O3) column having the diameter of a large pencil along with associated filters and tubing for obtaining Tc-99m
This apparatus is installed into radiation-shielded packages for shipment to Tc-99m suppliers. The generator includes both the package and its contained apparatus. Technetium generators can contain from 1 to 19 Ci of Mo-99, matched to address the needs and workloads of Tc-99m suppliers
It takes 18-24 hours to prepare technetium generators for shipment. Preparation involves loading the molybdate solution onto the columns and sterilizing them; installing the columns, tubing, and filters into the shielded generator package; and packaging the generators for shipment. Tc-99m generators are typically shipped to Tc-99m suppliers within a day of their manufacture. Generators are shipped in regulatory-compliant boxes. The delivery methods can be air, ground, or a combination of both depending on customer location and contracted transportation network.
The Mo-99 Market
The global medical community depends on a reliable supply of the radioisotope Mo-99 for nuclear medical diagnostic procedures. As previously described, Mo-99’s decay product, technetium-99m (Tc-99m), is used in over 40,000 medical procedures in the United States each day to diagnose heart disease and cancer, to study organ structure and function, and to perform other important medical applications.
In 2020, it is estimated (by Future Market Insights Inc, a global market research firm), that the Molybdenum 99 market generated revenues of approximately $3.8 billion. North America accounted for almost half of the Mo-99 demand. Approximately 62% of Mo-99 was used in hospitals while approximately 38% of Mo-99 use was in diagnostic centers.
73
The Mo-99 Supply Chain
The global Mo-99 supply chain is inherently fragile. The fragility stems primarily from two factors:
1. Mo-99 and its daughter isotope Tc-99m have short half-lives (66 and 6 hours, respectively) and therefore cannot be stockpiled. These radioisotopes need to be produced and delivered to the supply chain on a weekly or more frequent basis.
2. Global supply of Mo-99 currently relies on a small number of aging reactors worldwide and a small number of suppliers.
The current Mo-99 supply chain is also lengthy and prone to interruption throughout its course.
Recent Government Efforts to Increase Mo-99 Availability
Given the regular supply side shortages in the Mo-99 market, and widely anticipated shutdown of many of the current reactors, there is considerable focus on alternative methods of Tc-99m production. In 2012, Congress passed the American Medical Isotopes Production Act (AMIPA), which directed the National Nuclear Security Administration (NNSA) to establish a technology-neutral program to support the establishment of domestic supplies of Mo-99 without the use of HEU. NNSA has implemented this by competitively awarding 50%/50% cost-shared cooperative agreements to commercial entities and providing funds to the Department of Energy’s (DOE) National Laboratories to support development of non-HEU Mo-99 production technologies.
NNSA currently manages cooperative agreements with three U.S. companies, all developing diverse Mo-99 production technologies:
• NorthStar Medical Radioisotopes, LLC (Beloit, Wisconsin)
• Neutron capture technology using molybdenum-98 targets
• Accelerator-based technology using molybdenum-100 targets
• SHINE Technologies, LLC (Janesville, Wisconsin)
• Accelerator with fission technology to produce Mo-99 with an LEU solution target
• Niowave, Inc. (Lansing, Michigan)
• Superconducting electron linear accelerator with fission technology to produce Mo-99 with LEU targets
Mo-100 as an Alternative Intermediate to Produce Mo-99 and Tc-99m
Mo-100 is a stable isotope of molybdenum that does not decay. Naturally occurring molybdenum contains approximately 9.74% molybdenum-100. When highly enriched so that the Molybdenum contains >95% of the Mo-100 isotope, it can be used to produce either Mo-99 or Tc-99 via either photon-induced transmutation of Mo-100 into Mo-99 or via proton bombardment of Mo-100 into Tc-99m. The use of particle accelerators for the production of Mo-99 and direct production of Tc-99m has been studied extensively and the use of a particle accelerator conveys certain advantages and disadvantages. Accelerators produce ion beams and accelerate ions to higher energies by using oscillating electromagnetic fields. The accelerated particle beams have the capability of irradiating specific targets to produce Mo-99 and/or Tc-99m.
We intend to offer our Mo-100 to customers that may convert Mo-100 into Mo-99 or Mo-100 directly into Tc-99m. We believe that customers will be able to convert Mo-100 into Mo-99 using a cyclotron or a linear accelerator. The Mo-99 can then be converted into Tc-99m using a technetium generator. The technetium generators that are currently available will likely require some modifications in order to use the Mo-99 that has been produced via a cyclotron or a linear accelerator. These modifications will likely mean that new generator will require approval by healthcare regulators such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe.
74
Customers may convert Mo-100 directly into Tc-99m using a cyclotron, which would eliminate the need for a technetium generator. To date, only one healthcare regulator (Health Canada) has approved the use of Tc-99m that has been directly produced from Mo-100 in a low powered cyclotron. We believe it is likely that healthcare regulators in other countries will also require clinical data to support the use of Tc-99m that is produced directly from Mo-100.
We expect limited commercial activity for Mo-100 in the United States during the next two to three years and we anticipate that most of our initial revenues from future sales of our Mo-100 will be derived from countries in Asia and EMEA (Europe, Middle East and Africa).
Our Mo-100 Enrichment Facility
We are currently constructing a dedicated Mo-100 enrichment facility. The facility is located in Pretoria, South Africa and when complete, should be capable of enriching Mo-100 from its natural abundance of 9.7% to greater than 95%. At a level of 95% enrichment, the facility should be capable of producing 5 Kg of product initially. We expect to be able to expand the capacity of the plant for approximately $6m of capex and when expanded the plant should be capable of producing 20 Kg of enriched product (at 95% enrichment). A higher level of enrichment would result in a lower production capacity and a lower level of enrichment would result in a greater production capacity. It is likely that we can achieve premium selling prices at higher levels of enrichment.
Work on the plant originally started in 2016 but construction was halted in 2020 and 2021 due to a lack of funding. At that time, the plant was owned by a company called Molybdos (Pty) Limited. We acquired the assets of Molybdos during a business rescue process that involved a contested auction.
We currently expect the plant to be mechanically completed to an annual capacity of 5 Kg per year by the end of 2022. We currently expect to spend 2023 commissioning the plant and seeking regulatory approval with applicable regulators around the world.
ASP Technology for Uranium Enrichment
We believe our ASP technology is also capable of enriching Uranium, which we may be able to commercialize as a nuclear fuel component for use in the new generation of HALEU-fueled small modular reactors that are now under development for commercial and government uses.
Uranium is a naturally occurring element and is mined from deposits located in Kazakhstan, Canada, Australia, and several other countries including the United States. According to the World Nuclear Association (“WNA”), there are adequate measured resources of natural uranium to fuel nuclear power at current usage rates for about 90 years. In its natural state, uranium is principally comprised of two isotopes: uranium-235 (“U-235”) and uranium-238 (“U-238”). The concentration of U-235 in natural uranium is only 0.711%by weight. Most commercial nuclear power reactors require LEU fuel with a U-235 concentration greater than natural uranium and up to 5% by weight. Future reactor designs currently under development will likely require higher U-235 concentration levels of up to 20%. Uranium enrichment is the process by which the concentration of U-235 is increased (see discussion on HALEU demand below).
Separative work units (“SWU”) are a standard unit of measurement that represents the effort required to transform a given amount of natural uranium into two components: enriched uranium having a higher percentage of U-235 and depleted uranium having a lower percentage of U-235. The SWU contained in LEU is calculated using an industry standard formula based on the physics of enrichment. The amount of enrichment deemed to be contained in LEU under this formula is commonly referred to as its SWU component and the quantity of natural uranium deemed to be contained in LEU under this formula is referred to as its uranium or “feed” component. Currently, it is fairly common practice to purchase both the SWU and uranium components of LEU from the enrichment company. Therefore, LEU prices typically consist of two prices or components: SWU and uranium.
75
The following outlines the steps for converting natural uranium into LEU fuel, commonly known as the nuclear fuel cycle:
• Mining and Milling. Natural, or unenriched, uranium is removed from the earth in the form of ore and then crushed and concentrated.
• Conversion. Uranium ore concentrates (“UO”) are combined with fluorine gas to produce uranium hexafluoride (“UF”), a solid at room temperature and a gas when heated. UF is shipped to an enrichment plant.
• Enrichment. UF is enriched in a process that increases the concentration of the U isotope in the UF from its natural state of 0.711% up to 5%, or LEU, which is usable as a fuel for current light water commercial nuclear power reactors. Future commercial reactor designs may use uranium enriched up to 20% U, or HALEU.
• Fuel Fabrication. LEU is then converted to uranium oxide and formed into small ceramic pellets by fabricators. The pellets are loaded into metal tubes that form fuel assemblies, which are shipped to nuclear power plants. As the advanced reactor market develops, HALEU may be converted to uranium oxide, metal, chloride or fluoride salts, or other forms and loaded into a variety of fuel assembly types optimized for the specific reactor design.
• Nuclear Power Plant. The fuel assemblies are loaded into nuclear reactors to create energy from a controlled chain reaction. Nuclear power plants generate approximately 20% of U.S. electricity and 10% of the world’s electricity.
• Used Fuel Storage. After the nuclear fuel has been in a reactor for several years, its efficiency is reduced and the assembly is removed from the reactor’s core. The used fuel is warm and radioactive and is kept in a deep pool of water for several years. Many utilities have elected to then move the used fuel into steel or concrete and steel casks for interim storage.
The World is Transitioning to Newer Smaller Reactors
As the world transitions to a decarbonized electric grid, society is gradually decreasing its reliance on fossil fuels and increasing its reliance on “clean energy”. There appears to be bipartisan support for the growth of nuclear energy and the Biden Administration has identified carbon-free nuclear power as an essential part of achieving a net-zero CO2 economy by 2050. Nuclear power, through the operating light water reactor fleet and the deployment of advanced reactors, is poised to be an increasing contributor to carbon free energy in the U.S. and internationally. The United States leads the world in technology innovation with more developers of advanced reactors than any other country.
Small modular reactors (SMRs) are advanced nuclear reactors that have a power capacity of up to 300 MW(e) per unit, which is about one-third of the generating capacity of traditional nuclear power reactors. SMRs, which can produce a large amount of low-carbon electricity, are:
• Small — physically a fraction of the size of a conventional nuclear power reactor.
• Modular — making it possible for systems and components to be factory-assembled and transported as a unit to a location for installation.
• Reactors — harnessing nuclear fission to generate heat to produce energy.
Many of the benefits of SMRs are inherently linked to the nature of their design — small and modular. Given their smaller footprint, SMRs can be sited on locations not suitable for larger nuclear power plants. Prefabricated units of SMRs can be manufactured and then shipped and installed on site, making them more affordable to build than large power reactors, which are often custom designed for a particular location, sometimes leading to construction delays. SMRs offer savings in cost and construction time, and they can be deployed incrementally to match increasing energy demand.
76
In comparison to existing reactors, proposed SMR designs are generally simpler, and the safety concept for SMRs often relies more on passive systems and inherent safety characteristics of the reactor, such as low power and operating pressure. This means that in such cases no human intervention or external power or force is required to shut down systems, because passive systems rely on physical phenomena, such as natural circulation, convection, gravity and self-pressurization. These increased safety margins, in some cases, eliminate or significantly lower the potential for unsafe releases of radioactivity to the environment and the public in case of an accident.
SMRs have reduced fuel requirements. Power plants based on SMRs may require less frequent refueling, every 3 to 7 years, in comparison to between 1 and 2 years for conventional plants. Some SMRs are designed to operate for up to 30 years without refueling. SMRs are under construction or in the licensing stage in Argentina, Canada, China, Russia, South Korea and the United States of America.
Within the last five years significant legislation supporting the development and deployment of advanced reactors has been enacted: the Nuclear Innovation and Modernization Act, the Nuclear Energy Innovation and Capabilities Act, the Energy Act of 2020 and the Infrastructure Investment and Jobs Act. In addition, Congress established and funded the Advanced Reactor Demonstration Program which now supports two advanced reactor demonstrations to be deployed within seven years and eight other advanced reactor projects.
SMRs will require a different grade of enriched Uranium
Many advanced reactors, including the majority of the Advanced Reactor Demonstration Program awardees, will require High Assay Low Enriched Uranium (HALEU), and fuel forms very different from those manufactured for the current Light Water Reactors (LWRs). For example, the current generation of LWRs uses fuel enriched to less than 5% uranium-235. In contrast, many advanced non-LWR designs require enrichments between 5% and 20% with most above 10%.
Currently it is not possible to purchase HALEU between 10% and 20% from a commercial enricher in the United States. In the U.S., the infrastructure for the front-end of the fuel cycle for the utilization of low enriched uranium up to 5% U-235 is well defined. The U.S. has mining, conversion, enrichment, fabrication, and transportation capability. However, the infrastructure for producing and utilizing HALEU, in particular enrichments above 10%, is not established in the U.S. The mining and conversion infrastructure are common to all enrichment levels.
In 2020, the Department of Energy (DOE) selected two companies for awards under the Advanced Reactor Demonstration Program (ARDP) Pathway 1: Advanced Reactor Demonstrations. Both reactor designs require HALEU and can be operational in about seven years. Today, it is estimated that the companies selected for the demonstration pathway will require HALEU for their reactors beginning in 2024 to support fuel fabrication ahead of reactor startup. In addition, one of the companies under Pathway 2: Risk Reduction for Future Demonstrations will require HALEU in the 2024-2025 timeframe and other companies in Pathway 2 and 3 of the ARDP will also require HALEU. Privately funded companies are also working to deploy HALEU fueled reactors by the mid-2020s.
The Nuclear Energy Institute (NEI) believes that it is virtually impossible for HALEU to be provided to these companies in the needed quantities and timeframes from DOE inventories or commercial enrichers located in the U.S or Western Europe. Therefore, acquiring HALEU from other international suppliers will be required in the near term to support the larger goal of deploying advanced reactors in the U.S. in a timely manner. Deploying these reactors before 2030 will support climate goals and position the U.S. to be a strong exporter of advanced reactor technology. Per the recent NEI white paper, a robust domestic HALEU infrastructure is necessary to support both the domestic deployment of advanced reactors and the export of U.S. advanced reactor technologies requiring HALEU.
77
In a letter to the DOE captioned “Updated Need for High-Assay Low Enriched Uranium” dated December 20, 2021, the NEI provided an estimate of what U.S. HALEU demand may be during the next 15 years:
Estimated Annual Requirements for High Assay Low Enriched Uranium to 2035 (MTU/yr)
|
Company |
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
Total |
Cumulative |
||||||||||||
|
Year |
||||||||||||||||||||||||
|
2022 |
0.1 |
0.4 |
0.2 |
1.1 |
0.0 |
1.8 |
1.8 |
|||||||||||||||||
|
2023 |
0.1 |
3.1 |
4.4 |
0.1 |
7.7 |
9.5 |
||||||||||||||||||
|
2024 |
1.0 |
5.6 |
0.2 |
3.0 |
1.5 |
6.6 |
0.1 |
18.0 |
27.5 |
|||||||||||||||
|
2025 |
1.0 |
3.8 |
0.4 |
3.0 |
5.0 |
11.0 |
1.6 |
25.8 |
53.3 |
|||||||||||||||
|
2026 |
1.0 |
15.1 |
4.9 |
10.0 |
2.0 |
24.2 |
13.2 |
1.7 |
72.1 |
125.4 |
||||||||||||||
|
2027 |
1.0 |
26.5 |
7.9 |
4.0 |
24.2 |
13.2 |
1.9 |
78.7 |
204.1 |
|||||||||||||||
|
2028 |
1.0 |
37.8 |
16.6 |
13.0 |
23.0 |
24.2 |
13.2 |
2.0 |
130.8 |
334.9 |
||||||||||||||
|
2029 |
1.0 |
26.3 |
1.8 |
30.5 |
17.0 |
18.0 |
14.0 |
24.2 |
16.5 |
2.4 |
151.7 |
486.6 |
||||||||||||
|
2030 |
1.0 |
34.4 |
1.8 |
40.4 |
46.0 |
18.0 |
30.0 |
24.2 |
16.5 |
2.7 |
215.0 |
701.6 |
||||||||||||
|
2031 |
23.0 |
42.5 |
6.2 |
53.0 |
29.0 |
22.0 |
33.0 |
24.2 |
16.5 |
2.9 |
252.3 |
954.0 |
||||||||||||
|
2032 |
35.0 |
52.9 |
12.5 |
67.6 |
46.0 |
40.0 |
50.0 |
48.4 |
19.8 |
3.1 |
375.3 |
1329.2 |
||||||||||||
|
2033 |
47.0 |
63.5 |
32.2 |
82.1 |
46.0 |
32.0 |
80.0 |
48.4 |
19.8 |
3.2 |
454.2 |
1783.4 |
||||||||||||
|
2034 |
58.0 |
76.1 |
62.4 |
96.7 |
46.0 |
36.0 |
80.0 |
48.4 |
19.8 |
3.7 |
527.1 |
2310.5 |
||||||||||||
|
2035 |
70.0 |
90.9 |
96.0 |
112.4 |
91.0 |
29.0 |
50.0 |
48.4 |
22.0 |
4.1 |
613.8 |
2924.3 |
Notes:
• The material needs listed above are in metric tons of uranium per year and are a small amount compared to the approximately 2000 MTU used annually by the existing fleet of reactors.
• The material needs listed above include enrichments between 10.9 and 19.75% U-235.
• The year the material is needed is for fuel fabrication. Insertion in the reactor and reactor operations will occur in a later year.
• The material needs that are less than 1 MTU/year are for irradiation samples, lead test rods and lead test fuel assemblies.
• The material needs represent a few scenarios
• The deployment of an advanced fuel design for the existing fleet of light-water reactors.
• The deployment of multiple reactors of the same design that will not require refueling for many years.
• The deployment of reactors that have annual refueling requirements.
• These reactors include a range of sizes from a few Megawatt electric to 100s of Megawatt electric.
• The data above does not include utilities that are considering enrichment between 5% and 10%.
ASP Technology is ideally suited to the production of HALEU
We believe that we are in a very different position to many of the entrenched domestic and international enrichers. Our innovative isotope enrichment process has a number of advantages over traditional gas centrifuges and other novel approaches currently being explored by other companies: cheaper in Capex, faster in construction, more flexible in design and location.
78
We estimate that the capital cost of constructing an ASP plant for uranium enrichment is approximately 75% cheaper than that of a traditional gas centrifuge enrichment facility. Our manufacturing plants are modular, so our construction time is likely faster and more flexible than competing technologies. Our enrichment facilities are smaller than traditional gas centrifuges which means we can place them near fuel fabrication facilities for enhanced security of production and transportation. Our operating costs of enriching uranium to 15.5% - 19.75% U-235 should be comparable to or cheaper than costs for other methods of uranium enrichment.
The table below compares the ASP process with a traditional gas centrifuge when applied to a 20 mT plant.
|
ASP Plant |
Gas Centrifuge |
|||
|
Separation mechanism |
Stationary Wall Centrifuge |
Differential diffusion |
||
|
Capital Cost per plant |
<$150 million |
>$800 million |
||
|
Energy use (kWh) per SWU |
<500 |
50-240 |
||
|
Construction time |
2-3 years |
2-3 years |
||
|
Levelized cost per SWU* |
$65 |
$140 |
____________
* for enrichment from 0.71% U235 to 5% U235
We are currently conducting a feasibility study with respect to constructing an enrichment facility in either the United States or an international location. Construction of a new ASP enrichment facility in US would be done in three phases. The first phase would involve the construction and validation of an ASP test bench, the engineering design of the first segment and obtaining required permits and licenses from regulators. Excluding the licensing process, we expect this phase would take approximately 9-12 months.
The second phase would involve the construction of the first segment and control systems for the plant and the engineering design of the additional stages. We expect this stage would take approximately 9-12 months, resulting in the plant capable of operating in a close-loop setup which would demonstrate enrichment and start to produce small quantities of enriched Uranium.
The third phase would involve the construction of the remaining segments that will complete the plant and the commissioning phase. We expect this phase would take approximately 20-30 months and the production volume would gradually ramp up to the final capacity of 20 metric tons per year. Importantly, subject to licensure, we can produce commercial quantities of HALEU by 2026 that would satisfy the anticipated demand from all the advanced reactor currently in development. We can supply HALEU at a price lower than the HALEU currently imported from international enrichers and considerably lower than any potential domestic supply that may evolve.
Much of the control systems, compressors and hardware used in a uranium enrichment plant would be identical or similar to parts used to construct our Molybdenum plant in Pretoria. Our molybdenum plant uses molybdenum hexafluoride (MoF6) and a Uranium pant would use uranium hexafluoride (UF6).
Intellectual Property
Our business will depend on the proprietary ASP technology licensed by us from Klydon. To date, we and Klydon have relied exclusively on trade secrets and other intellectual property laws, non-disclosure agreements with our respective employees, consultants, vendors, potential customers and other relevant persons and other measures to protect our intellectual property, and intend to continue to rely on these and other means. As we intend to transition into the commercialization of Mo-100, we envision our intellectual property and its security becoming more vital to our future. Pursuing patent protection remains part of the intellectual property protection philosophy and strategy and the advisability of establishing provisional patent rights is continuously assessed on a case-by-case basis in respect of both conceptual aspects and the specific applications thereof. Such assessments are made in consultation with regulatory bodies and with due consideration to the prospects of successfully obtaining patent protection in light of any disclosure constraints that are imposed by such bodies.
Recent Developments
On October 25, 2022, our outside counsel received a letter (the “NMS Letter”) from a law firm acting on behalf of Norsk Medisinsk Syklotronsenter AS (“NMS”), asserting, among other things, that the grant of a license to the ASP technology for the separation of isotopes of Molybdenum that have medical applications to ASP Isotopes Inc. by Klydon violates a pre-existing exclusive sub-license to the ASP technology granted to Radfarma, as more
79
fully described below. NMS and ASP Isotopes had previously been briefly in discussions regarding a potential future collaboration on technology and product development. However, these preliminary discussions have not been active for several months, after death of a lead NMS scientist and ASPI decision to explore other options.
The NMS Letter makes reference to: (1) a license agreement entered into on October 25, 2013 by Klydon and API Labs Pharmaceuticals (Proprietary) Limited (“API Labs”) to license the ASP technology for enriching certain isotopes of the element Molybdenum (“2013 API Labs License”); and (2) an exclusive sub-license to the ASP technology granted on October 1, 2019 to Radfarma, as licensee, by API Labs and SaPhotonica Limited (“SaPhotonica”), as licensors (the “2019 Radfarma Sub-License”). The NMS Letter states that Radfarma is a joint venture that is 45% owned by NMS and 45% owned by SaPhotonica.
The NMS Letter asserts, among other things, that the grant of a license to the ASP technology to ASP Isotopes Inc. by Klydon violates a covenant in the 2019 Radfarma Sub-License that the licensors shall not be entitled, directly or indirectly, to use, grant or otherwise give the rights, or any similar rights, which were granted to Radfarma under the 2019 Radfarma Sub-License to any other person for use in the territory. The sub-license granted to Radfarmain the 2019 RadfarmaSub-License related to the use of intellectual property rights related to the ASP technology for the separation of isotopes of Molybdenum that have medical applications. “Territory” is defined in the 2019 Radfarma Sub-License as “the Kingdom of Norway for the construction of the 20-kilogram capacity plants; and means the international market where distribution agreements can be produced.” The NMS Letter asserts that while Klydon purported to give to ASP Isotopes Inc. a license to market the ASP technology globally, these rights were already granted to Radfarma.
The NMS Letter includes a request for ASP Isotopes Inc. to enter into discussions for an agreement with NMS based on terms proposed in previous correspondence from NMS. The previous correspondence from NMS included the following key prerequisites to a possible cooperation between NMS and ASP Isotopes Inc.:(1) NMS will be granted the right to set up an enrichment facility for a Mo-100 in Norway; (2) NMS will be granted the exclusive rights to sales, marketing, and distribution in Europe, while ASPI gets similar rights for the rest of the world; and (3) NMS will support the development of the Mo-100 target production and Mo-99 generator production and if required the sales operations of ASPI.
The NMS Letter does not include a threat of litigation against ASP Isotopes Inc. or any parties to the 2013 API Labs License or 2019 Radfarma Sub-License. However, if the licensed rights granted to us are found to be invalid or unenforceable (in whole or in part), or if our exclusive license agreement with Klydon is terminated or Klydon, as licensor, fails to abide by the terms of our exclusive license agreement, our ability to commercialize our future isotopes would suffer and our business, results of operations and financial condition may be adversely affected. If the prior sub-license granted to Radfarmais found to be valid, we would be required to cease using the ASP technology for the separation of isotopes of Molybdenum that have medical applications (unless we were able to obtain a license from Radfarma), and we would focus our business operations on the enrichment of isotopes other than Molybdenum.For example, instead of continuing to pursue the production of Molybdenum-100, we could focus on the production and commercialization of zinc, silicon and/or chlorine using the ASP technology. We expect that our Mo-100 plant in South Africa would need to be redesigned and retrofitted in order to produce other isotopes, which would take approximately six months and cost approximately $1 million. See “Risk Factors — Risks Related to Our Intellectual Property” — “We have received a letter asserting that the license for the ASP technology granted to us from Klydon, which is critical to our business, may be invalid because these rights were already granted to a third party, Radfarma” and “Our license for the ASP technology with Klydon may be found to infringe third party intellectual property rights.”
Based on information currently available, and after consultation with legal counsel in South Africa, management believes that our exclusive license for the enrichment of Molybdenum-100 and all other isotopes from Klydon are valid and the company will vigorously defend its rights.
Regulatory Environment
We are subject to a variety of laws and regulations, including but not limited to those of the United States and South Africa, that impose regulatory systems that govern many aspects of our operations, including our research and development activities involving the enrichment of isotopes in South Africa. In addition, these jurisdictions impose trade controls requirements that restrict trade to comply with applicable export controls and economic sanctions laws and requirements, and legal requirements that are intended to curtail bribery and corruption.
There are a number of regulators and treaties that govern and control our business and industry. The two principal ones that control and regulate the manufacturing of isotopes at our isotope enrichment facility in South Africa are the International Atomic Energy Agency (IAEA) and the Nuclear Non-Proliferation Treaty (NPT).
80
The IAEA is an international organization that seeks to promote the peaceful use of nuclear energy, and to inhibit its use for any military purpose, including nuclear weapons. The IAEA was established as an autonomous organization on 29 July 1957. Though established independently of the United Nations through its own international treaty, the IAEA Statute, the IAEA reports to both the United Nations General Assembly and Security Council. The IAEA statute currently has 173 member states, including South Africa.
The IAEA is authorized to conclude agreements with member states, in terms of which agreements the agency would perform certain functions and the relevant member states would be placed under certain obligations. The IAEA has concluded an extensive suite of agreements with South Africa. These agreements can be viewed on the website of the IAEA (https://www.iaea.org/resources/legal/country-factsheets) and include agreements that govern the physical protection of nuclear material, the notification of nuclear accidents, assistance in the case of nuclear accidents, nuclear safety, civil liability, and technical cooperation.
The Treaty on the Non-Proliferation of Nuclear Weapons, commonly known as the Non-Proliferation Treaty or NPT, is an international treaty whose objective is to prevent the spread of nuclear weapons and weapons technology, to promote cooperation in the peaceful uses of nuclear energy, and to further the goal of achieving nuclear disarmament and general and complete disarmament. Our South African subsidiary is registered with the South African Council for the Non-Proliferation of Weapons of Mass Destruction in terms of the Non-Proliferation of Weapons of Mass Destruction Act, 1993. Our registration certificate is valid until September 3, 2023. Representatives from the South African Council for the Non-Proliferation of Weapons of Mass Destruction regularly inspect our facility and conduct tests to monitor the activities that are taking place at our facilities.
In South Africa, government Notice 493 relates to nuclear-related dual-use equipment, materials and software and related technologies which can be used in their entirety or in part for the separation of uranium isotopes. ASP is classified as a dual use technology under the protocols of the IAEA and, as such, is subject to the controls that are implemented under these protocols. These controls comprise requirements that include:
• membership of the IAEA and adherence to its protocols;
• membership of the Nuclear Suppliers Group (NSG) and adherence to its protocols;
• agreement to an “additional protocol” in light of uranium enrichment capabilities;
• local laws that requires permits for possession, operation and commercialization and regular reporting;
• ad hoc inspections by the IAEA on 24 hour and in some cases 2 hours pre-warning;
• requirement for proposed patent applications to be approved at ministerial level; and
• cross-border technology transfer to be handled by the respective governments and approved by IAEA.
These regulations place strict limitations on what we can and cannot do. Security measures at our production facility and our offices are stringent. Access to our manufacturing plant is highly controlled. All employees and all visitors to the manufacturing plant are prescreened by the South African Council for the Non-Proliferation of Weapons of Mass Destruction before being allowed employment or entry into the facility. Some of our suppliers also need to be registered with the South African Council for the Non-Proliferation of Weapons of Mass Destruction. Many of our computer systems are not connected to the external internet and confidential information is secured at a controlled location.
Currently, the production, distribution or sale of Mo-100 is not regulated by a healthcare regulator such as the Food and Drug Administration (FDA) in the USA, Health Canada in Canada, the European Medicines Agency in Europe and similar regulators in other countries. However, products that are produced from Mo-100 (such as Mo-99 and Tc-99m in a linear accelerator or cyclotron) are regulated by healthcare regulators and our customers are required to operate under the licensure of these healthcare regulators. Currently, the production and use of Tc-99m from Mo-100 in a cyclotron is only approved in one country (Canada).
Some of our future isotopes may also be regulated by healthcare regulators such as the Food and Drug Administration (FDA) in the USA, Health Canada in Canada, the European Medicines Agency in Europe and similar regulators in other countries.
U.S. laws restrict the ability of U.S. companies, U.S. citizens and U.S. permanent residents, or U.S. persons, from involvement in certain types of transactions with countries, businesses and individuals that have been targeted by U.S. economic sanctions. For example, U.S. persons are precluded from undertaking virtually any activity of
81
any kind on the part of any U.S. person with regard to any potential or actual transactions involving Cuba, Iran and Sudan without the prior approval of the U.S. Department of Treasury’s Office of Foreign Assets Control, or OFAC. OFAC also administers U.S. sanctions against a lengthy list of entities and individuals, wherever they may be located, that the United States considers to be closely associated with these sanctioned countries or that are considered terrorists or traffickers in either narcotics or weapons of mass destruction. Furthermore, U.S. economic sanctions forbid U.S. persons from circumventing direct U.S. restrictions or from facilitating transactions by non-U.S. persons if those activities are forbidden to U.S. persons. Penalties for violating provisions such as these can include significant civil and criminal fines, imprisonment and loss of tax credits or export privileges.
The Foreign Corrupt Practices Act of 1977, or the FCPA, as amended by the Omnibus Trade and Competitiveness Act of 1988 and the International Anti-Bribery and Fair Competition Act of 1998, makes it a criminal offense for a U.S. corporation or other U.S. domestic concern to make payments, gifts or give anything of value directly or indirectly to foreign officials for the purpose of obtaining or retaining business, or to obtain any other unfair or improper advantage. In addition, the FCPA imposes accounting standards and requirements on publicly traded U.S. corporations and their foreign affiliates, which are intended to prevent the diversion of corporate funds to the payment of bribes and other improper payments, and to prevent the establishment of “off books” slush funds from which such improper payments can be made. We are also subject to laws and regulations covering subject matter similar to that of the FCPA that have been enacted by countries outside of the United States. For example, the Convention on Combating Bribery of Foreign Public Officials in International Business Transactions was signed by the members of the Organization for Economic Cooperation and Development and certain other countries in December 1997. The Convention requires each signatory to enact legislation that prohibits local persons and firms from making payments to foreign officials for the purpose of obtaining business or securing other unfair advantages from foreign governments. Failure to comply with these laws could subject us to, among other things, penalties and legal expenses, which could harm our reputation and have a material adverse effect on our business, financial condition and results of operations.
Compliance with the myriad of export control laws of the various jurisdictions in which we do business is a challenge for any company involved in export activities within the nuclear and defense end markets. We have compliance systems in our U.S. and non-U.S. subsidiaries to identify those products and technologies that are subject to export control regulatory restrictions and, where required, we obtain authorization from relevant regulatory authorities for sales to foreign buyers or for technology transfers to foreign consultants, companies, universities or foreign national employees. We also have a compliance system that is intended to proactively address potential compliance issues including those related to export control, trade sanctions and embargoes, as well as anti-bribery situations, and we are implementing this through such mechanisms as training, formalizing contracting processes, performing diligence on agents and continuing to improve our record-keeping and auditing practices with respect to third-party relationships and otherwise. Thus far, as part of our compliance system, for instance, we have developed a Code of Ethics and Conduct that informs all of our employees of their compliance obligations. Furthermore, we have developed an ethics and conduct training program that all of our employees are required to undertake, as well as other targeted compliance training relevant to their position, such as specific FCPA training for all of our worldwide controllers. Violations of any of the various U.S. or non-U.S. export control laws can result in significant civil or criminal penalties, or even loss of export privileges, as mentioned above. We recognize that an effective compliance program can help protect the reputation and relationship of a regulated company with the regulatory agencies administering these laws and regulations. In the United States, each of the regulatory agencies administering these laws and regulations has a voluntary disclosure program that offers the possibility of significantly reduced penalties, if any are applicable, and we intend to use these programs as part of our overall compliance program, as necessary.
Employees
As of September 30, 2022, we employed four employees. None of our employees are represented by a labor union. We consider our relationship with our employees to be good.
Facilities
We lease our research and development facility in Pretoria, South Africa under a lease with a term expiring on December 31, 2030. We believe that our existing facilities are adequate to meet our current needs.
Legal Proceedings
We are currently not a party to any material legal proceedings.
82
Executive Officers and Directors
Set forth below is certain biographical and other information regarding our directors and our executive officers.
|
Name |
Age |
Position(s) |
||
|
Executive Officers |
||||
|
Paul E. Mann |
46 |
Chairman and Chief Executive Officer |
||
|
Hendrik Strydom, Ph.D. |
62 |
Chief Technology Officer and Director |
||
|
Robert Ainscow |
46 |
Interim Chief Financial Officer |
||
|
Non-Employee Directors |
||||
|
Joshua Donfeld |
46 |
Director |
||
|
Duncan Moore, Ph.D. |
63 |
Director |
||
|
Sergey Vasnetsov |
58 |
Director |
||
|
Todd Wider, M.D. |
57 |
Director |
The following are brief biographies describing the backgrounds of our executive officers and directors.
Executive Officers
Paul E. Mann co-founded our company in September 2021 and has served as our Chairman and Chief Executive Officer and a member of our board of directors since incorporation. Paul also served as our Chief Financial Officer until September 2022. Prior to ASP Isotopes, Paul was Chief Financial Officer of PolarityTE, Inc. (Nasdaq: PTE), a biotechnology company, from June 2018 until April 2020. Prior to that, he responsible for Healthcare investments at DSAM Partners LLC, a global hedge fund. Earlier in his career, he was a portfolio manager at Highbridge Capital where he managed investments in healthcare and biotechnology. Prior to Highbridge Capital, from August 2013 to March 2016, he worked at Soros Fund Management where he was responsible for billions of dollars of investments in healthcare and chemicals companies. During his career as a healthcare and chemicals investor, Paul has helped create and fund numerous early stage and start-up companies. Prior to moving to the buy-side, Paul spent 11 years as a sell-side analyst at Morgan Stanley and Deutsche Bank. He co-managed the healthcare research team at Morgan Stanley, one of the top ranked teams in Institutional Investor, Greenwich and Reuters. He was also corporate broker to over half the UK Pharmaceutical Companies. Paul started his career as a research scientist at Procter and Gamble and he is named as the inventor of numerous skin creams in the Oil of Olay range of cosmetics. He is also a nonexecutive, independent director at Abeona Therapeutics (NASDAQ: ABEO), where he is the chair of the audit committee, and a director at Healthtech Solution Inc. (OTC: HLTT), where he is chairman of the board and serves on the audit committee. He is the co-founder and Chairman of Varian Biopharma, a private biotechnology company focused on precision oncology. Paul has an MA (Cantab) and an MEng from Cambridge University, UK where he studied Natural Sciences and Chemical Engineering and he is a CFA charter holder.
We believe Mr. Mann’s detailed knowledge and unique perspective and insights as our founder and Chief Executive Officer, as well as his prior experience as Chief Financial Officer of another public company and extensive experience managing investments in healthcare, biotechnology and chemicals companies, qualify him to serve on our board of directors and position him well to serve as our Chairman.
Robert Ainscow co-founded our company in September 2021 and served as our VP and Head of Business Development until September 2022 when he was appointed Interim Chief Financial Officer. Prior to ASP Isotopes, Robert was head of capital markets at Zenzic Partners Limited from October 2017 to February 2021 and a founder of Bluezest Mortgages since November 2015. Robert has over 20 years’ experience in financing operating companies and lending platforms through the provision of structured finance and securitisation structures in the debt capital markets. He has developed, executed and managed innovative structures to fund credit, renewable energy and transport and logistics assets encompassing all major financial jurisdictions, on and off-shore. Robert began his career at the first ever internet-bank, First-E; in the investment banking division, WIT-Soundview. Following the “.com” correction he entered mainstream investment banking at U.S. firms Morgan Stanley and Bear Stearns in London where he was an analyst in the Law Division with responsibility for capital markets oversight and a
83
Vice President in the Principal and Asset-Backed Finance Group with responsibility for securitisation respectively. He subsequently worked at Investec bank twice over the subsequent years as well as a variety of directorships, consultancies and investments in start-up and growth phase lending and securitisation platforms.
Hendrik Strydom, Ph.D. has served has served as our Chief Technology Officer since January 2022 and has served on our board of directors since January 2022. Dr. Strydom co-developed the isotope separation technology, known as “Aerodynamic Separation Process” (ASP). In 1993 Dr. Strydom co-founded SDI Ltd (now named Klydon), a research and development company which developed the ASP. Klydon, where Dr. Strydom currently serves as CEO, successfully exploited the ASP technology by separating Silicon (Si28), Carbon (C13 & C14), Oxygen (O-18) and Molybdenum (Mo-100). Since the commencement of commercial operation of the O-18 plant over 3 years ago, Klydon continues to sell O-18 into the South African radio pharmacy market. Dr. Strydom’s work on separation of isotopes started when he was employed as a scientist at the South African Atomic Energy Corporation (AEC), where he specialized in the laser separation of heavy isotopes. Dr. Strydom left AEC in 1993 to co-found Klydon. Dr. Strydom holds a BSc- Hons (Physics & Maths) (1983) — University of Pretoria, MSc (Physics) (1990) — University of Port Elizabeth, PhD (Physics) (2000) — University of Natal (Durban).
As the founder and CEO of Klydon, Dr. Strydom brings to the Board his detailed knowledge and unique perspective and insights regarding the strategic and operational opportunities and challenges, economic and industry trends, and competitive and financial positioning of our business.
Non-Employee Directors
Joshua Donfeld has served on our board of directors since October 2021. Joshua was most recently (May 2016-October 2020) a co-founding and co-managing partner of Castle Hook Partners, a New York-based investment management fund. At Castle Hook, among other responsibilities, he was responsible for overseeing the fund’s equity investments in sectors such as healthcare and natural resources. Prior to Castle Hook, Mr. Donfeld was a portfolio manager at Soros Fund Management from May 2012-April 2016. At Soros he was responsible for managing a portfolio of assets across public and private investments in industries spanning Energy, Utilities, Materials, Industrials, Healthcare, Consumer, Infrastructure and Technology. Prior to Soros Joshua was a Managing Director at Canyon Partners in Los Angeles where he was responsible for the firm’s Energy and Utilities investments in credit, distressed and equities. Mr. Donfeld has extensive experience in early-stage investing and he has extensive experience in capital markets, capital structuring, business planning, strategic planning, Wall Street management, corporate finance and accounting. Mr. Donfeld graduated Magna Cum Laude from Princeton University with a BA in Economics and a focus on Chinese language/East Asian Studies.
The Board believes that Mr. Donfeld’s significant financial expertise and experience contribute to the Board’s understanding and ability to analyze complex issues, particularly as the Company looks to grow its business, and qualify him to serve on our board of directors.
Duncan Moore, Ph.D. has served on our board of directors since October 2021. Duncan is a partner at East West Capital Partners since May 2008, which has a focus on making investments in the Healthcare Industry in Asia. Previously, from 1991 to 2008, Dr. Moore was a top-ranked pharmaceutical analyst at Morgan Stanley leading the firm’s global healthcare equity research team. Whilst at the University of Cambridge, he co-founded a medical diagnostics company called Ultra Clone with two colleagues which led to the beginnings of a 20-year career in healthcare capital markets analysis. In 1986, he was involved in setting up the BankInvest biotechnology funds and was on its scientific advisory board. Dr. Moore was educated in Edinburgh and went to the University of Leeds where he studied Biochemistry and Microbiology. He has a M.Phil. and Ph.D. from the University of Cambridge where he was also a post-doctoral research fellow. Currently, he is an active investor in biomedical companies as Chairman of Lamellar Biomedical and Allarity Therapeutics A/S (previously Oncology Venture A/S). In addition, he has a board position at Forward Pharma A/S, Cycle Pharma and GH Research. Duncan is the Chairman of the Scottish Life Sciences Association.
We believe that the experience, insights and knowledge Dr. Moore possesses from his leadership roles in business activities are important qualifications, skills and experience that provide valuable assistance to the Board and greatly contribute to the overall knowledge of the Board and its ability to address the issues we confront.
84
Sergey Vasnetsov has served on our board of directors since October 2021. Sergey has a long history in the chemicals industry as both a senior executive and as an investor. Since June 2016 he has been is the founder and managing partner of ChemBridges LLC, a strategy consulting firm for chemicals companies. From August 2010 to May 2016 Sergey was Senior Vice President Strategic Planning and Transactions and a member of the Executive Leadership Team for LyondellBasell (NYSE: LYB). His responsibilities included long-range financial and strategic planning, capital investments, external and internal benchmarking, cost reduction and profit enhancement programs. Prior to joining LyondellBasell, Sergey was Managing Director, Head of Global Chemicals Equity Research at Barclays Capital and Lehman Brothers. Sergey started his career as a Senior Research Chemist in Catalyst R&D at Union Carbide where he was the author of 8 US and World patents. Sergey has a Master of Science in Catalysis from the University of Novosibirsk, Russia. He was a George Soros Scholar at Oxford University (UK) and later earned an MBA in finance from Rutgers University.
We believe Mr. Vasnetsov’s experience and knowledge in the chemicals industry, acquisitions and general business matters, and his demonstrated leadership roles in other business activities are important qualifications, skills and experience that benefits the Board.
Todd Wider, M.D. has served on our board of directors since October 2021. Dr. Wider is the Executive Chairman and Chief Medical Officer of Emendo Biotherapeutics, which focuses on highly specific and differentiated next generation gene editing. Dr. Wider served on the board of directors of ARYA Sciences Acquisition Corp I, which had a successful business combination with Immatics N.V. (IMTX) in 2020. He served on the board of ARYA Sciences Acquisition Corp II, which had a successful business combination with Nautilus Biotechnology (NAUT) in 2021. He also served on the board of ARYA III, which had a successful business combination with Cerevel Therapeutics (CERE) in 2021. He is also on the boards of ARYA Sciences Acquisition Corp IV and V (ARYD and ARYE), Abeona Therapeutics Inc. (Nasdaq: ABEO), Varian Biopharma, Xanadu Bio, and Lyfebulb. Dr. Wider previously consulted with a number of entities in the biotechnology space. Dr. Wider is an active, honorary member of the medical staff of Mount Sinai Hospital in New York, where he worked for over 20 years, focused on reconstructive surgery. Dr. Wider received an MD from Columbia College of Physicians and Surgeons, where he was Rudin Fellow, and an AB, with high honors and Phi Beta Kappa, from Princeton University. He did his residency in general surgery and plastic and reconstructive surgery at Columbia Presbyterian Medical Center, and postdoctoral fellowships in complex reconstructive surgery at Memorial Sloan Kettering Cancer Center, where he was Chief Microsurgery Fellow, and in craniofacial surgery at the University of Miami. Dr. Wider is also a principal in Wider Film Projects, a documentary film company focused on producing films with sociopolitical resonance that have won Academy, Emmy and Peabody Awards.
We believe Dr. Wider, as a result of his vast public and private company board experience at a variety of companies, possesses knowledge and experience in various areas, including business leadership, finance and technology, which strengthens the Board’s overall knowledge, capabilities and experience.
Board Composition
Our bylaws provide that our board of directors shall initially consist of six members, and thereafter shall be fixed from time to time by resolution of our board of directors. Currently our board of directors consists of six members: Paul Mann, Joshua Donfeld, Duncan Moore, Hendrik Strydom, Sergey Vasnetsov and Todd Wider.
In accordance with our Certificate of Incorporation, our board of directors will be divided into three classes with staggered three year terms. At each annual meeting of stockholders after the initial classification, the successors to the directors whose terms will then expire will be elected to serve from the time of election and qualification until the third annual meeting following their election. Our directors will be divided among the three classes as follows:
• the Class I directors will be Paul Mann and Joshua Donfeld, and their terms will expire at the annual meeting of stockholders to be held in 2023;
• the Class II directors will be Sergey Vasnetsov and Duncan Moore, and their terms will expire at the annual meeting of stockholders to be held in 2024; and
• the Class III directors will be Hendrik Strydom and Todd Wider, and their terms will expire at the annual meeting of stockholders to be held in 2025.
85
Any increase or decrease in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of our board of directors may have the effect of delaying or preventing changes in control of our company.
Our board of directors has determined that, upon completion of this offering, Messrs. Donfeld, Moore and Wider will be independent directors. In making this determination, our board of directors applied the standards set forth in the rules of Nasdaq and in Rule 10A-3 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Our board of directors considered all relevant facts and circumstances known to it in evaluating the independence of these directors, including their current and historical employment, any compensation we have given to them, any transactions we have with them, their beneficial ownership of our capital stock, their ability to exert control over us, all other material relationships they have had with us and the same facts with respect to their immediate family. We intend to rely on a phase-in period under these rules applicable to newly public companies, which will permit fewer than a majority of our board of directors to be independent on the listing date of our common stock, provided we satisfy such requirement within one year of the date of listing. Accordingly, we intend to have a majority of our board of directors consist of independent directors within one year of the date our common stock is listed on The Nasdaq Capital Market.
Although there is no specific policy regarding diversity in identifying director nominees, both the Nominating and Corporate Governance Committee and the board of directors seek the talents and backgrounds that would be most helpful to us in selecting director nominees. In particular, the Nominating and Corporate Governance Committee, when recommending director candidates to the full board of directors nomination, may consider whether a director candidate, if elected, assists in achieving a mix of board of directors members that represents a diversity of background and experience.
Board Leadership Structure
Our board of directors recognizes that one of its key responsibilities is to evaluate and determine its optimal leadership structure so as to provide effective oversight of management. Our bylaws and corporate governance guidelines, will provide our board of directors with flexibility to combine or separate the positions of Chairman of the Board and Chief Executive Officer. Our board of directors currently believes that our existing leadership structure, under which Paul E. Mann serves as our chief executive officer, is effective, provides the appropriate balance of authority between independent and non-independent directors, and achieves the optimal governance model for us and for our stockholders.
Board Oversight of Risk
Although management is responsible for the day to day management of the risks our company faces, our board of directors and its committees take an active role in overseeing management of our risks and have the ultimate responsibility for the oversight of risk management. The board of directors regularly reviews information regarding our operational, financial, legal and strategic risks. Specifically, senior management attends quarterly meetings of the board of directors, provides presentations on operations including significant risks, and is available to address any questions or concerns raised by our board of directors.
In addition, we expect that our three committees will assist the board of directors in fulfilling its oversight responsibilities regarding risk. The Audit Committee will coordinate the Board of Director’s oversight of our internal control over financial reporting, disclosure controls and procedures, related party transactions and code of conduct and management will regularly report to the Audit Committee on these areas. The Compensation Committee will assist the board of directors in fulfilling its oversight responsibilities with respect to the management of risks arising from our compensation policies and programs. The Nominating and Corporate Governance Committee will assist the board of directors in fulfilling its oversight responsibilities with respect to the management of risks associated with board organization, membership and structure, succession planning for our management and directors and corporate governance. When any of the committees receives a report related to material risk oversight, the chairman of the relevant committee will report on the discussion to the full board of directors.
86
Code of Business Conduct and Ethics
We have adopted a written code of business conduct, that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Upon the closing of this offering, a current copy of the code will be posted on the Investor Relations section of our website at www.aspisotopes.com. The information contained on our website is not part of this prospectus. If we make any substantive amendments to, or grant any waivers from, the code of business conduct and ethics for any officer or director, we will disclose the nature of such amendment or waiver on our website or in a current report on Form 8-K within four business days of such amendment or waiver.
Board Committees
Our board of directors has established an audit committee, or the Audit Committee, a compensation committee, or the Compensation Committee, and a nominating and corporate governance committee, or the Nominating and Corporate Governance Committee, each of which will operate pursuant to a charter to be adopted by our board of directors and will be effective upon the closing of this offering. Our board of directors may also establish other committees from time to time to assist the board of directors. Effective upon the closing of this offering, the composition and functioning of all of our committees will comply with all applicable requirements of the Sarbanes-Oxley Act and Nasdaq and SEC rules and regulations. Upon our listing on the Nasdaq, each committee’s charter will be available on our website at www.aspisotopes.com.
Audit Committee
The members of our Audit Committee are Todd Wider, Joshua Donfeld and Duncan Moore, with Mr. Wider serving as chair. Our board of directors has determined that each member of the Audit Committee is “independent” as that term is defined in the SEC and Nasdaq rules, meets the heightened independence requirements for audit committees required under Section 10A of the Exchange Act and related SEC and Nasdaq rules, and has sufficient knowledge in financial and auditing matters to serve on the Audit Committee. Our board of directors has designated Mr. Donfeld as an “audit committee financial expert,” as defined under the applicable rules of the SEC. The audit committee’s responsibilities include:
• appointing, approving the compensation of and assessing the independence of our independent registered public accounting firm;
• pre-approving auditing and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm;
• reviewing the overall audit plan with our independent registered public accounting firm and members of management responsible for preparing our financial statements;
• reviewing and discussing with management and our independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us;
• coordinating the oversight and reviewing the adequacy of our internal control over financial reporting;
• establishing policies and procedures for the receipt and retention of accounting-related complaints and concerns;
• recommending based upon the audit committee’s review and discussions with management and our independent registered public accounting firm whether our audited financial statements shall be included in our Annual Report on Form 10-K;
• monitoring the integrity of our financial statements and our compliance with legal and regulatory requirements as they relate to our financial statements and accounting matters;
• preparing the audit committee report required by SEC rules to be included in our annual proxy statement;
• reviewing all related person transactions for potential conflict of interest situations and approving all such transactions; and
• reviewing quarterly earnings releases.
87
Compensation Committee
The members of our Compensation Committee are Duncan Moore and Todd Wider with Mr. Moore serving as chair. Our board of directors has determined that each member of the Compensation Committee is “independent” as that term is defined in SEC and Nasdaq rules, meets the heightened independence requirements for compensation committee purposes under Section 10C of the Exchange Act and related SEC and Nasdaq rules, and is a ”non-employee director” under Rule 16b-3 under the Exchange Act. The compensation committee’s responsibilities include:
• reviewing and approving our philosophy, policies and plans with respect to the compensation of our chief executive officer;
• making recommendations to our board of directors with respect to the compensation of our chief executive officer and our other executive officers;
• reviewing and assessing the independence of compensation advisors;
• overseeing and administering our equity incentive plans;
• reviewing and making recommendations to our board of directors with respect to director compensation; and
• preparing the Compensation Committee reports on executive compensation, including a “Compensation Discussion and Analysis” disclosure, for inclusion in the company’s proxy statement for the annual meeting of shareholders, if and when applicable in accordance with rules and regulations of the SEC.
Nominating and Corporate Governance Committee
Effective upon the closing of this offering Joshua Donfeld, Duncan Moore and Sergey Vasnetsov will serve on the Nominating and Corporate Governance Committee, which will be chaired by Mr. Donfeld. We intend to rely on the phase-in rules of Nasdaq with respect to the requirement that the nominating and corporate governance committee be composed entirely of members of our board of directors who satisfy the standards of independence established for independent directors under the Nasdaq rules, as determined by our board of directors. Our board of directors has determined that Messrs. Donfeld and Moore are “independent” as defined in Nasdaq rules. The Nominating and Corporate Governance Committee’s responsibilities include:
• developing and recommending to the board of directors criteria for board and committee membership;
• establishing procedures for identifying and evaluating board of director candidates, including nominees recommended by stockholders;
• reviewing the composition of the board of directors to ensure that it is composed of members containing the appropriate skills and expertise to advise us;
• identifying and screening individuals qualified to become members of the board of directors;
• recommending to the board of directors the persons to be nominated for election as directors and to each of the board’s committees;
• developing and recommending to the board of directors a code of business conduct and ethics; and
• overseeing the evaluation of our board of directors and management.
Compensation Committee Interlocks and Insider Participation
None of the members of our Compensation Committee has during the prior fiscal year been one of our officers or employees or had a relationship requiring disclosure under “Certain Relationships and Related Party Transactions.” None of our executive officers currently serves, or in the past fiscal year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
88
This section discusses the material components of the executive compensation program for Paul Mann, our Co-Founder and Chairman and Chief Executive Officer (and former Chief Financial Officer), who we refer to as our “named executive officer.” We only have one named executive officer for fiscal year 2021 because our company has a small number of executive officers and no other executive officer of our company received compensation in excess of $100,000 for fiscal year 2021.
This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt following the closing of this offering may differ materially from the currently planned programs summarized in this discussion.
Summary Compensation Table
The following table sets forth information concerning the compensation of our named executive officer for the fiscal year ended December 31, 2021.
|
Name and Principal Position |
Salary |
Bonus |
Stock |
Option |
Non-Equity |
All Other |
Total |
||||||||||||||
|
Paul Mann |
$ |
60,000 |
$ |
— |
$ |
375,000 |
$ |
— |
$ |
— |
$ |
— |
$ |
435,000 |
|||||||
|
Chairman and Chief Executive Officer(1) |
|
|
|
|
|
|
|
||||||||||||||
____________
(1) Mr. Mann served as our Chairman, Chief Executive Officer since September 2021. He also served as our Chief Financial Officer from September 2021 until September 2022.
(2) In accordance with SEC rules, these columns reflect the aggregate grant date fair value of the restricted stock awards granted during 2021. This amount has been computed in accordance with Financial Accounting Standards Board (FASB), Accounting Standards Codification (ASC) Topic 718. Assumptions used in the calculation of this amount are described in Note 2 to our audited consolidated financial statements included elsewhere in this prospectus. This amount does not reflect the actual economic value that will be realized by Mr. Mann upon the vesting of the stock awards or the sale of the common stock underlying such awards.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information regarding outstanding option awards held by our named executive officer as of December 31, 2021.
|
Option Awards |
Stock Awards |
||||||||||||||
|
Name |
Number of |
Number of |
Option |
Option |
Number of |
Market |
|||||||||
|
Paul Mann |
— |
— |
— |
— |
1,500,000 |
(1) |
$ |
3,000,000 |
(2) |
||||||
____________
(1) The amounts reported in this column represent 1,500,000 shares of performance-based restricted common stock granted by us to Mr. Mann in October 2021. The shares vest upon achieving certain performance conditions and market conditions upon the third anniversary of the date of grant.
(2) This amount reflects the fair market value of our common stock of $2.00 per share as of December 31, 2021 (the determination of the fair market value by our board of directors as of the most proximate date) multiplied by the amount shown in the column for the number of shares that have not vested.
89
Employment Agreements
Below is a description of our employment agreements with, Paul Mann, our named executive officer for fiscal year 2021, including a discussion of the severance pay and other benefits to be provided in connection with a termination of employment and/or a change in control under the arrangements with our named executive officer. Additionally, below is a description of our employment agreement with Robert Ainscow, our current Interim Chief Financial Officer.
Paul Mann.
We entered into an executive employment agreement with Mr. Mann in October 2021, which governs the current terms of his employment with us as Chief Executive Officer. Pursuant to the agreement, Mr. Mann is entitled to an annual base salary of $240,000 per annum for the first six months and $480,000 per annum for the remainder of the employment period, a target annual discretionary bonus equal to 100% of his annual base salary, and milestone-based bonuses paid in shares of our common stock based on the achievement of revenue milestones. Annual bonuses will be paid in a mixture of cash and common stock, as determined by the compensation committee.
Subject to our achievement of $4.167 million in average monthly revenues for a trailing three- month period Mr. Mann will be paid a $1,000,000 bonus. Subject to our achievement of $8.33 million in average monthly revenues for a trailing three-month period Mr. Mann will be paid an additional $1,000,000 bonus. Subject to our achievement of $12.5 million in average monthly revenues for a trailing three-month period Mr. Mann will be paid an additional $1,000,000 bonus. Subject to our achievement of $16.67 million in average monthly revenues for a trailing three-month period Mr. Mann will be paid an additional $1,000,000 bonus. Any earned milestone-based bonuses will be paid within 30 days of the achievement of the applicable revenue goal and the number of vested shares issued to Mr. Mann shall be determined by dividing the $1,000,000 bonus amount by either the then fair market value per share of common stock, as determined in good faith by our board of directors, or the closing sale price of our common stock on the trading day immediately preceding the applicable payment date, as reported by the principal trading market for our common stock.
Mr. Mann’s employment agreement has an initial term of three years and will automatically renew for successive one year periods unless either party provides notice of termination. Mr. Mann is also entitled to certain severance benefits under the terms of his employment agreement.
Upon a termination of Mr. Mann’s employment for any reason, Mr. Mann is entitled to receive a pro-rata annual bonus for the year of termination.
Upon a termination of Mr. Mann’s employment for any reason other than due to his voluntary resignation without good reason and which does not occur in connection with a change in control, Mr. Mann will receive continued payment of Mr. Mann’s base salary until the end of the then-applicable remaining employment period term and reimbursement of COBRA premiums for up to an 18-month period.
Upon a termination of Mr. Mann’s employment due to his death, disability, termination without cause, resignation for good reason, or resignation in connection with a change of control, the vesting and exercisability of all equity awards held by Mr. Mann shall immediately accelerate, so that all such equity awards shall be fully vested and exercisable as of the date of his termination. Additionally, upon such termination Mr. Mann’s stock options (as well as any other exercisable equity awards) will remain exercisable until the earlier one year after Mr. Mann’ s termination or the original maximum permitted term of the equity award.
Robert Ainscow.
We also entered into an executive employment agreement with Robert Ainscow in October 2021 pursuant to which he was appointed as Vice President and Head of Business Development. We entered into an amendment to Mr. Ainscow’s employment agreement in September 2022 in connection with his appointment as Interim Chief Financial Officer. Pursuant to the agreement (as amended), Mr. Ainscow is entitled to an initial base salary of $160,000 per annum (which will increase to $300,000 per annum when the company has produced 250 grams of commercial product), a target annual discretionary bonus equal to 40% of his annual base salary, and milestone-based bonuses paid in shares of our common stock based on the achievement of revenue milestones. Annual bonuses will be paid in a mixture of cash and common stock, as determined by the compensation committee.
90
Mr. Ainscow’s employment agreement has an initial term of one year and will automatically renewed for successive one year periods unless either party provides notice of termination. Mr. Ainscow is also entitled to certain severance benefits under his employment agreement.
Upon a termination of Mr. Ainscow’s employment for any reason other than due to his voluntary resignation without good reason and which does not occur in connection with a change in control, Mr. Ainscow will receive reimbursement of COBRA premiums for up to an 18-month period.
Upon a termination of Mr. Ainscow’s employment due to his death, disability, or termination without cause, resignation for good reason, or resignation in connection with a change in control the vesting and exercisability of all equity awards held by Mr. Ainscow shall immediately accelerate, so that all such equity awards shall be fully vested and exercisable as of the date of his termination. Additionally, upon such termination Mr. Ainscow’s stock options (as well as any other exercisable equity awards) will remain exercisable until the earlier of one year after Mr. Ainscow’ s termination or the original maximum permitted term of the equity award.
Performance Share Award.
On October 4, 2021 Mr. Mann was awarded 1,500,000 shares of performance-based restricted common stock. The number of shares that may become vested and nonforfeitable will range for zero to 1,500,000 and be determined on the third anniversary of the grant (or, if earlier, the date on which a change in control transaction occurs), which is the “measurement date,” based on the “adjusted share price” on the measurement date, which will be calculated based on the average of the adjusted closing prices of our common stock during the 90 consecutive trading days ending on the specified measurement date, divided by the total number of shares common stock outstanding and underlying any convertible preferred stock outstanding as of the measurement date. If, on the measurement date, the “adjusted share price” (a) is less than $0.25, then all shares subject to the award will be immediately forfeited for no consideration; (b) equals or exceeds $0.50, then 20% of the shares subject to the award will vest on the measurement date; (c) equals or exceeds $0.75, then 40% of the shares subject to the award will vest on the measurement date, (d) equals or exceeds $1.00, then 60% of the shares subject to the award will vest on the measurement date, (e) equals or exceeds $1.25, then 80% of the shares subject to the award will vest on the measurement date; and (f) equals or exceeds $1.50, then 100% of the shares subject to the award will vest on the measurement date. The shares subject to the award may also become vested and nonforfeitable before the measurement date; if the adjusted share price exceeds any of the prices described in foregoing clauses (a)-(f) for 90 consecutive trading days before the measurement date, then the corresponding number of shares subject to the award will immediately vest.
2022 Plan Awards at IPO.
We intend to make special one-time awards of restricted stock upon completion of this offering to our officers, directors and consultants in recognition of their contributions to our initial public offering process and their ongoing service to us as a public company. All of these awards are contingent upon completion of this offering. The shares of restricted stock are subject to time-based vesting and are not subject to further performance-based criteria. Our named executive officer, Mr. Mann, will receive 1,000,000 shares of restricted stock and Mr. Vasnetsov and Mr. Ainscow will each receive 600,000 shares of restricted stock. Each of our directors (excluding Messrs. Mann and Vasnetsov) will receive 200,000 shares of restricted stock. The restricted stock awards to our executives and consultants will become 25% vested on each anniversary of this offering and the restricted stock awards to our directors (other than Messrs. Mann and Vasnetsov) will become 50% vested on each of the first and second anniversary of this offering, subject to continuous service of the participant with us through each such date.
Employee Benefit and Equity Incentive Plans
2022 Equity Incentive Plan
In October 2022 our board of directors adopted, and our stockholders approved, the 2022 Plan, which will become effective immediately prior to the closing of this offering. We intend to use the 2022 Plan following the closing of this offering to provide incentives that will assist us to attract, retain, and motivate employees, including officers, consultants, and directors.
91
We may provide these incentives through the grant of stock options, stock appreciation rights, restricted stock, RSUs, performance shares, and units and other cash-based or share-based awards. In addition, the 2022 Plan contains a mechanism through which we may adopt a deferred compensation arrangement in the future.
A total of 5,000,000 shares of our common stock are initially authorized and reserved for future issuance under the 2022 Plan. This reserve will automatically increase on January 1, 2023 and each subsequent anniversary through 2032, by an amount equal to the smaller of:
• 5.0% of the number of shares of common stock issued and outstanding on the immediately preceding December 31; and
• an amount determined by our board of directors.
Appropriate adjustments will be made in the number of authorized shares and other numerical limits in the 2022 Plan and in outstanding awards to prevent dilution or enlargement of participants’ rights in the event of a stock split or other change in our capital structure. Shares subject to awards which expire or are cancelled or forfeited will again become available for issuance under the 2022 Plan.
The shares available under the 2022 Plan will not be reduced by awards settled in cash. Shares withheld or reacquired by us in satisfaction of our tax withholding obligations pursuant to the exercise or settlement of options or SARs or the vesting or settlement of full value equity awards shall again become available for issuance under the 2022 Plan. The gross number of shares issued upon the exercise of stock appreciation rights or options exercised by means of a net exercise or by tender of previously owned shares will be deducted from the shares available under the 2022 Plan.
The 2022 Plan generally will be administered by the compensation committee of our board of directors, which we refer to as the administrator. Subject to the provisions of the 2022 Plan, the compensation committee will determine in its discretion the persons to whom and the times at which awards are granted, the sizes of such awards and all of their terms and conditions. The compensation committee will have the authority to construe and interpret the terms of the 2022 Plan and awards granted under it. The 2022 Plan provides, subject to certain limitations, for indemnification by us of any director, officer, or employee against all reasonable expenses, including attorneys’ fees, incurred in connection with any legal action arising from such person’s action or failure to act in administering the 2022 Plan.
In any one year period measured commencing on the date of our annual meeting of stockholders for a particular year that is held following the closing of our initial public offering and ending on the day immediately prior to the date of our annual meeting of stockholders for the next subsequent year, the maximum number of shares of common stock subject to stock awards granted under the 2022 Plan or otherwise during any period to any non-employee director, taken together with any cash fees paid by us to such non-employee director during such period for service on the board of directors, will not exceed $600,000 in total value (calculating the value of any such stock awards based on the grant date fair value of such stock awards for financial reporting purposes), or, with respect to the period in which a non-employee director is first appointed or elected to our board of directors, $1,000,000.
The 2022 Plan will authorize the compensation committee, without further stockholder approval, to provide for the cancellation of stock options or stock appreciation rights with exercise prices in excess of the fair market value of the underlying shares of common stock on the date of grant in exchange for new options or other equity awards with exercise prices equal to the fair market value of the underlying common stock on the date of grant or a cash payment or other full value equity awards.
Awards may be granted under the 2022 Plan to our employees, including officers, directors, or consultants or those of any present or future parent or subsidiary corporation or other affiliated entity. All awards will be evidenced by a written agreement between us and the holder of the award and may include any of the following:
• Stock options. We may grant non-statutory stock options or incentive stock options (as described in Section 422 of the Code), each of which gives its holder the right, during a specified term (not exceeding ten years) and subject to any specified vesting or other conditions, to purchase a number of shares of our common stock at an exercise price per share determined by the administrator, which may not be less than the fair market value of a share of our common stock on the date of grant.
92
• Stock appreciation rights. A stock appreciation right, or SAR, gives its holder the right, during a specified term (not exceeding ten years) and subject to any specified vesting or other conditions, to receive the appreciation in the fair market value of our common stock between the date of grant of the award and the date of its exercise. We may pay the appreciation in shares of our common stock or in cash.
• Restricted stock. The administrator may grant restricted stock awards either as a bonus or as a purchase right at a price determined by the administrator. Shares of restricted stock remain subject to forfeiture until vested, based on such terms and conditions as the administrator specifies. Holders of restricted stock will have the right to vote the shares and to receive any dividends paid, except that the dividends may be subject to the same vesting conditions as the related shares.
• Restricted stock units. Restricted stock units, or RSUs, represent rights to receive shares of our common stock (or their value in cash) at a future date without payment of a purchase price, subject to vesting or other conditions specified by the administrator. Holders of RSUs have no voting rights or rights to receive cash dividends unless and until shares of common stock are issued in settlement of such awards. However, the administrator may grant RSUs that entitle their holders to dividend equivalent rights.
• Performance awards. Performance awards, consisting of either performance shares or performance units, are awards that will result in a payment to their holder only if specified performance goals are achieved during a specified performance period. The administrator establishes the applicable performance goals based on one or more measures of business performance, such as revenue, gross margin, net income or total stockholder return. To the extent earned, performance awards may be settled in cash, in shares of our common stock or a combination of both in the discretion of the administrator. Holders of performance shares or performance units have no voting rights or rights to receive cash dividends unless and until shares of common stock are issued in settlement of such awards. However, the administrator may grant performance shares that entitle their holders to dividend equivalent rights.
• Cash-based awards and other share-based awards. The administrator may grant cash-based awards that specify a monetary payment or range of payments or other share-based awards that specify a number or range of shares or units that, in either case, are subject to vesting or other conditions specified by the administrator. Settlement of these awards may be in cash or shares of our common stock, as determined by the administrator. Their holders will have no voting rights or right to receive cash dividends unless and until shares of our common stock are issued pursuant to the awards. The administrator may grant dividend equivalent rights with respect to other share-based awards.
In the event of a change in control as described in the 2022 Plan, the acquiring or successor entity may assume or continue all or any awards outstanding under the 2022 Plan or substitute substantially equivalent awards. The compensation committee may provide for the acceleration of vesting of any or all outstanding awards upon such terms and to such extent as it determines, except that the vesting of all awards held by members of the board of directors who are not employees will automatically be accelerated in full. Any awards that are not assumed, continued, or substituted for in connection with a change in control or are not exercised or settled prior to the change in control will terminate effective as of the time of the change in control. The 2022 Plan will also authorize the compensation committee, in its discretion and without the consent of any participant, to cancel each or any outstanding award denominated in shares upon a change in control in exchange for a payment to the participant with respect to each share subject to the cancelled award of an amount equal to the excess of the consideration to be paid per share of common stock in the change in control transaction over the exercise price per share, if any, under the award.
The 2022 Plan will continue in effect until it is terminated by the compensation committee, provided, however, that all awards will be granted, if at all, within ten years of its effective date. The compensation committee may amend, suspend or terminate the 2022 Plan at any time, provided that without stockholder approval, the plan cannot be amended to increase the number of shares authorized, change the class of persons eligible to receive incentive stock options, or effect any other change that would require stockholder approval under any applicable law or listing rule.
93
2021 Stock Incentive Plan
The 2021 Plan was originally adopted by our board of directors in October 2021 and was amended by our board of directors in August 2022. The maximum aggregate number of shares of common stock that may be issued under the 2021 Plan is 7,000,000. Upon the closing of this offering, our board of directors will terminate the 2021 Plan and we will not grant any further awards under such plan, but the 2021 Plan will continue to govern outstanding awards granted thereunder. Our compensation committee administers the 2021 Plan and has the authority, among other things, to construe and interpret the terms of the 2021 Plan and awards granted thereunder, and is referred to as the administrator of the 2021 Plan in this summary.
The 2021 Plan permits the grant of options. As of June 30, 2022, we had options to purchase 2,266,000 shares of common stock outstanding under the 2021 Plan. Appropriate and proportionate adjustments will be made to the number of shares subject to outstanding awards to prevent dilution or enlargement of participants’ rights in the event of a recapitalization, reclassification, stock dividend, stock split, reverse stock split, split-up, split-off, spin-off, combination of shares, exchange of shares or similar change in our capital structure, or in the event of payment of a dividend or distribution to our stockholders in a form other than shares (excepting normal cash dividends). All awards will be evidenced by a written agreement between us and the holder of the award and may include any of the following:
• Stock options. We may grant non-statutory stock options or incentive stock options (as described in Section 422 of the Code), each of which gives its holder the right, during a specified term (not exceeding ten years) and subject to any specified vesting or other conditions, to purchase a number of shares of our common stock at an exercise price per share determined by the administrator, which may not be less than the fair market value of a share of our common stock on the date of grant.
• Restricted stock awards. We may grant restricted stock awards either as a bonus or as a purchase right at such price as the administrator determines. Shares of restricted stock remain subject to forfeiture until vested, based on such terms and conditions as the administrator specifies. Holders of restricted stock will have the right to vote the shares and to receive any dividends paid, except that the dividends will be subject to the same vesting conditions as the related shares.
• Restricted stock units. RSUs represent rights to receive shares of our common stock (or their value in cash) at a future date without payment of a purchase price, subject to vesting or other conditions specified by the administrator. Holders of RSUs have no voting rights or rights to receive cash dividends unless and until shares of common stock are issued in settlement of such awards. However, the administrator may grant RSUs that entitle their holders to dividend equivalent rights.
In its discretion, our compensation committee may provide for acceleration of the exercisability, vesting or settlement of awards in connection with a “change in control,” as defined under the 2021 Plan, of each or any outstanding award or portion thereof and common stock acquired pursuant thereto upon such conditions, including termination of the plan participant’s service prior to, upon or following such change in control, and to such extent as our compensation committee determines. In the event of a change in control, the surviving, continuing, successor or purchasing corporation or other business entity or parent thereof, as the case may be, may, without the consent of any plan participant, either assume or continue the rights and obligations under each or any award or portion thereof outstanding immediately prior to the change in control or substitute for each or any such outstanding award or portion thereof a substantially equivalent award with respect to the stock of the surviving, continuing, successor or purchasing corporation or other business entity or parent thereof, as applicable. Any award or portion thereof which is neither assumed nor continued by the surviving, continuing, successor or purchasing corporation or other business entity or parent thereof in connection with the change in control nor exercised or settled as of the time of consummation of the change in control shall terminate and cease to be outstanding effective as of the time of consummation of the change in control.
94
Limitation of Liability and Indemnification
Our Certificate of Incorporation will contain provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
• any breach of the director’s duty of loyalty to us or our stockholders;
• any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
• unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law, or the DGCL; or
• any transaction from which the director derived an improper personal benefit.
Our Certificate of Incorporation and our Bylaws will provide that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Our Certificate of Incorporation and our Bylaws will also provide that we may indemnify a director, officer, employee or agent (including the advancement of the final disposition of any action or proceeding), and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under Delaware law. We have entered and expect to continue to enter into agreements to indemnify and advance expenses to our directors, executive officers and other employees as determined by our board of directors. With specified exceptions, these agreements provide for indemnification for related expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that these Bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain directors’ and officers’ liability insurance.
The limitation of liability and indemnification provisions in our Certificate of Incorporation and our Bylaws may discourage stockholders from bringing a lawsuit against our directors and officers for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and our stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage.
Director Compensation
The following table sets forth information regarding compensation earned by our non-employee-directors for service on our board of directors during the year ended December 31, 2021. Hendrik Strydom, Ph.D. joined our board of directors in January 2022.
|
Name |
Fees Earned |
Stock |
All Other |
Total |
||||
|
Josh Donfeld |
— |
— |
— |
— |
||||
|
Duncan Moore, Ph.D. |
— |
— |
— |
— |
||||
|
Sergey Vasnetsov |
— |
— |
— |
— |
||||
|
Todd Wider, M.D. |
— |
— |
— |
— |
We have entered into director agreements with Messrs. Donfeld, Moore and Wider, pursuant to which we agreed to pay to each such director a fee for his service of $60,000 per year, payable at the director’s discretion in cash or common stock at market value. The initial payment will be made on October 13, 2022 provided that the director continues to serve through such date. Following the initial payment, the fee will be paid quarterly in arrears ($15,000 quarterly instalments) on the last business day of each December, March, June and September during the director’s term. In addition, we agreed to award a common stock award with a market value of $100,000 on October 13, 2022 and annually each year thereafter during the director’s term. Directors who are also our employees will not receive fees for service on our board of directors.
95
2022 Plan Awards at IPO. We intend to make special one-time awards of restricted stock upon completion of this offering to our officers, directors and consultants in recognition of their contributions to our initial public offering process and their ongoing service to us as a public company. All of these awards are contingent upon completion of this offering. The shares of restricted stock are subject to time-based vesting and are not subject to further performance-based criteria. Each of our directors (excluding Messrs. Mann and Vasnetsov) will receive 200,000 shares of restricted stock. The restricted stock awards to our directors (other than Messrs. Mann and Vasnetsov) will become 50% vested on each of the first and second anniversary of this offering, subject to continuous service of the participant with us through such date. Mr. Vasnetsov will receive 600,000 shares of restricted stock which will become 25% vested on each anniversary of this offering, subject to continuous service of the participant with us through each such date.
96
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than the compensation agreements and other arrangements described in the “Executive Compensation” section of this prospectus and the transactions described below, since September 13, 2021, the date of our incorporation, there has not been and there is not currently proposed, any transaction or series of similar transactions to which we were, or will be, a party in which the amount involved exceeded, or will exceed, $120,000 and in which any director, executive officer, holder of 5% or more of any class of our capital stock or any member of the immediate family of, or entities affiliated with, any of the foregoing persons, had, or will have, a direct or indirect material interest.
Our Relationship with Klydon Proprietary Limited (“Klydon”)
Dr Einar Ronander, who serves as Chief Scientific Adviser to our board of directors, and Dr Hendrik Strydom, one of our directors, previously co-founded and currently serve as Executive Chairperson and Chief Executive Officer, of Klydon. Dr Ronander and Dr Strydom are the controlling shareholders of Klydon through Isotope Separation Technology (Pty) Ltd, a company jointly owned by Dr Ronander and Dr Strydom and the largest shareholder of Klydon. Dr Ronander and Dr Strydom each own approximately 11.9% of our outstanding shares of common stock. Immediately following the closing of this offering, Dr Ronander and Dr Strydom will each own 11.4% of our outstanding shares of common stock (or approximately 11.3% of our common stock, if the underwriters exercise in full their option to purchase additional shares of our common stock in this offering). As a result, Dr Ronander and Dr Strydom will continue to have significant influence over our business, including pursuant to the agreements described below. The agreements summarized below are filed as exhibits to the registration statement of which this prospectus is a part, and the summaries of these agreements set forth the terms of the agreements that we believe are material. These summaries are qualified in their entirety by reference to the full text of such agreements.
Exclusive Mo-100 License (superseded and replaced by new license (see “Omnibus Klydon License” below)). On September 30, 2021, our subsidiary, ASP Isotopes South Africa (Proprietary) Limited (“ASP South Africa”), as licensee, entered into a license with Klydon, as licensor, pursuant to which ASP South Africa acquired from Klydon an exclusive license to use, subcontract and sublicense certain intellectual property rights relating to the ASP technology for the development and/or otherwise disposing of the ASP technology and production, distribution, marketing and or sale of Mo-100 isotope produced using the ASP technology (as amended on June 8, 2022, the “Mo-100 license”). The intellectual property rights granted to us through the Mo-100 license included all existing and/or future proprietary rights of Klydon relating to the ASP technology, whether or not such rights have been registered including the copyright, designs, know-how, patents and trademarks (although Klydon currently has no such patents, patent applications or copyrights). The exclusive Mo-100 license was royalty-free, had a term of 999 years and was for the global development of the ASP Technology and production of the Mo-100 Isotope and global for the distribution, marketing and sale of the Mo-100 Isotope. No upfront or other payment was made or is owed in connection with the Mo-100 license. Klydon had the right to terminate the exclusivity of the Mo-100 license in the event that the licensee ceased carrying on activities of Mo-100 enrichment for a period of greater than 24 consecutive months. Klydon had no other rights to terminate the Mo-100 license. Effective July 26, 2022, the parties agreed to terminate the Mo-100 license, which was superseded and replaced by a new license agreement (described under the heading “Omnibus Klydon License” below).
Exclusive U-235 License (superseded and replaced by new license (see “Omnibus Klydon License” below)). On January 25, 2022, ASP South Africa, as licensee, entered into a license with Klydon, as licensor, pursuant to which ASP South Africa acquired from Klydon an exclusive license to use, subcontract and sublicense certain intellectual property rights relating to the ASP technology for the development and/or otherwise disposing of the ASP technology and production, distribution, marketing and or sale of U-235 produced using the ASP (as so amended, the “U-235 license”). The exclusive U-235 license was for the global development of the ASP technology and production of U-235 and global for the distribution, marketing and sale of U-235. In connection with the U-235 license we made an upfront payment of $100,000 and agreed to pay certain royalties (the greater of $50 per k.g. of U-235 and 10% of profits) and a 33% sublicensing revenue share of any cash consideration we may receive for any sublicenses we may grant. Klydon had the right to terminate the exclusivity of the U-235 license in the event that the licensee ceased carrying on activities of U-235 enrichment for a period longer than 24 consecutive months. Klydon had no other rights to terminate the U-235 license. Effective July 26, 2022, the parties agreed to terminate the U-235 license, which was superseded and replaced by a new license agreement (described under the heading “Omnibus Klydon License” below).
97
Omnibus Klydon License. On July 26, 2022, ASP Isotopes UK Ltd, as licensee, entered into a license agreement with Klydon, as licensor, pursuant to which ASP Isotopes UK Ltd acquired from Klydon an exclusive license to use, develop, modify, improve, subcontract and sublicense certain intellectual property rights relating to the ASP technology for the production, distribution, marketing and sale of all isotopes produced using the ASP technology (the “Klydon license agreement”). The intellectual property rights granted to us through the Klydon license agreement include all existing and/or future proprietary rights of Klydon relating to the ASP technology, whether or not such rights have been registered including the copyright, designs, know-how, patents and trademarks (although Klydon currently has no such patents, patent applications or copyrights). The Klydon license agreement superseded and replaced the Mo-100 license and U-235 license described above. The Klydon license agreement is royalty-free, has a term of 999 years and is worldwide for the development of the ASP technology and the distribution, marketing and sale of isotopes. Future production of isotopes is limited to member countries of the Nuclear Suppliers Group. In connection with the Klydon license agreement we agreed to make an upfront payment of $100,000 (to be included within the payments we make under the Turnkey Contract (described below) and deferred payments of $300,000 over 24 months. Klydon has the right to terminate the exclusivity of the Klydon license agreement in the event that the licensee ceases to carry on activities related to isotope enrichment for a period longer than 24 consecutive months.
Turnkey Contract. On November 1, 2021, ASP South Africa and Klydon, as the contractor, entered into a contract under which Klydon has been appointed to supply to ASP South Africa a complete turnkey Molybdenum-100 enrichment plant (the “Turnkey Contract”). The activities to be undertaken or performed by Klydon include: taking control of the assets acquired in the Molybdos Business Rescue Auction; the design of a Molybdenum-100 enrichment facility with target manufacturing capability of 20 Kg p.a of 95% and above enriched Molybdenum isotope; the supply of components, equipment and labor required for 20 Kg p.a.; the installation, testing and commissioning of the Molybdenum enrichment plant, including production of targets to be used by customers in cyclotrons; securing all required approvals, regulatory authorizations and other required consents for the operation of the plant; providing training to local ASP Isotopes South Africa (Proprietary) Limited personnel to enable them to operate the plant going forward; and providing warranties in relation to the performance targets of the plant which are required to be met. Klydon will be responsible for liaising with the relevant South African authorities including the South African Non Proliferation Council, the Nuclear Suppliers Group and International Atomic Energy Agency to ensure that the Turnkey Contract and the Molybdenum-100 enrichment plant are compliant with international laws and guidelines. The consideration to be paid by ASP Isotopes South Africa (Proprietary) Limited under the Turnkey Contract is a maximum of $12.8 million, in the following stages: (1) $6.8 million in an initial proof of concept stage (which stage will end at the point of first production of Mo-100); and (2) $6.0 million for increasing production capacity through modular construction (from the expected initial capacity of 5 kg p.a. to 20 kg p.a. of 95% enriched molybdenum-100). The Company’s management expects that the initial proof of concept stage (Phase 1) will be completed during the second half of 2022 and an additional 12 months will be needed for completion of the secondary investment stage (Phase 2).
Letter of Intent for Klydon Shares or Assets. On September 30, 2021, ASP South Africa entered into a letter of intent with Klydon and Isotope Separation Technology (Pty)Ltd (Klydon’s largest shareholder which is owned by Dr Ronander and Dr Strydom) with respect to the acquisition of all of the outstanding shares or substantially all of the assets of Klydon. Under the letter of intent (as amended), Klydon has agreed to negotiate with us on an exclusive basis. We are in the process of preparing, and negotiating with Klydon, the share purchase agreement and related agreements with respect to the Klydon acquisition, but such transaction documents are not yet in agreed form and as of the date hereof, several issues remain open that, if not resolved, will prevent us from entering into a definitive agreement with respect to the Klydon acquisition. We do not expect the timing or success of the Klydon acquisition to have a material effect on either our business or our financial results in the future because of the existing commercial agreements that we have with Klydon. We believe that the Klydon license agreement and the Turnkey Contract provide us with the requisite intellectual property rights and personnel (through Klydon’s workforce) that we need to conduct our business as currently proposed to be conducted. While an acquisition of Klydon would be beneficial to us in terms of adding employees in South Africa, the services of the individuals who are working to deliver the Mo-100 enrichment plant are already assured under the Turnkey Contract with Klydon. In the event we do not complete an acquisition of Klydon by the completion of the Turnkey Contract (after Klydon has delivered a fully commissioned Mo-100 enrichment plant), we would likely need to enter into a new agreement with Klydon as a contractor in order to operate the new Mo-100 enrichment plant. Alternatively, we would need to hire employees who would be able to operate the new Mo-100 enrichment plant.
98
Acquisition of Silicon-28 Plant Assets. On July 26, 2022, we acquired assets comprising a dormant Silicon-28 aerodynamic separation processing plant from Klydon for ZAR 6,000,000 (which at the then current exchange rate was approximately USD 364,000), which will be payable to Klydon on the later of 180 days of the acquisition and the date on which the assets generate any revenues of any nature.
Chief Scientific Adviser Agreement with Dr Ronander. In January 2022, we entered into an agreement with Dr Einar Ronander pursuant to which he agreed to serve as chief scientific adviser to the board of directors for quarterly payments of $50,000. The agreement has an initial term of one year and will automatically renew for successive one year periods unless either party provides notice of termination.
Consulting Agreements with Dr Strydom and Dr Ronander. In January 2022, we entered into consulting agreements with Dr Einar Ronander, who serves as Chief Scientific Adviser to our board of directors, and Dr Hendrik Strydom, one of our directors, pursuant to which each of Dr Ronander and Dr Strydom agreed to assist us in developing the ASP technology for the enrichment of uranium and potentially forming a licensing transaction relating to the enrichment of uranium. In addition, Dr Ronander agreed to assist us obtaining all regulatory approvals and permits for the company’s operations. The consulting agreements had no upfront cash payment or regular payment but provide for cash payments to the consultants in the event that a licensing upfront payment is paid to the company in connection with any type of licensing transaction relating to the enrichment of uranium, with the amount of such cash payments to the consultants to be determined based upon the date of receipt of any such licensing upfront payment: 25% of any licensing upfront payment received within 3 months will be paid to the consultants (75% retained by the company), 15% of any licensing upfront payment received between 3 – 9 months will be paid to the consultants (85% retained by the company), and 5% of any licensing upfront payment received after 9 months will be paid to the consultants (95% retained by the company). The consulting agreements have no fixed term but either party may terminate the consulting agreement (i) without cause upon 30 days’ written notice to the other party or (ii) effective immediately upon written notice to the other party, if the other party breaches the agreement (subject to a 10-day cure period if such breach is capable of cure).
Indemnification Arrangements with Drs Ronander and Strydom. In connection with the other agreements entered into with Dr Einar Ronander and Dr Hendrik Strydom in January 2022, we have agreed to indemnify each of Dr Ronander and Dr Strydom against any and all losses, damages, liabilities, deficiencies, claims, actions, judgments, settlements, interest, awards, penalties, fines, costs, or expenses of whatever kind, including professional fees and reasonable attorneys’ fees, that are incurred by the indemnitees, arising out of any claim by a third party creditor related to an agreement such third party creditor entered into with Klydon, Dr Einar and Dr Strydom and Klydon, and Isotope Separation Technology (Pty) Ltd (the largest shareholder of Klydon, which is owned by Dr Ronander and Dr Strydom) in May 2012 related to, among other things, (i) the sale of shares in Isotope Separation Technology (Pty) Ltd by such third party creditor to Dr Ronander and Dr Strydom and (ii) the acknowledgment of certain loan obligations owed by Klydon to Isotope Separation Technology (Pty) Ltd and such third party creditor and the repayment terms for such loan obligations. Our indemnification obligations under the letter agreements with Dr Ronander and Dr Strydom are subject to a maximum aggregate liability of $3,200,000 (which is approximately the amount that may be owed to the third party creditor). We are aware of the possibility of claims by the third party creditor related to the failure by Klydon to make repayment of certain loan obligations under this May 2012 agreement, but no such claim or litigation has been asserted or threatened. We do not believe Klydon, Isotope Separation Technology (Pty) Ltd or any other third party is obligated to provide indemnity against any such claim. We do not believe any payment obligation under our indemnification arrangements with Dr Ronander and Dr Strydom is currently probable.
Advisor Agreement with ChemBridges LLC
We have entered into an Advisor Agreement with ChemBridges LLC dated October 27, 2021. One of our directors, Sergey Vasnetsov, is the President and owner of ChemBridges LLC. Under the Advisor Agreement, ChemBridges LLC agreed to provide subject matter expertise on a wide range of commercial activity and strategic execution of key global business objectives, including but not limited to the advisory services on strategy, M&A, R&D, organic growth, operational optimization, commercial excellence, IR and corporate governance. Compensation under the Advisor Agreement includes (i) an initial grant of 600,000 shares of restricted common stock that vest annually over three years and (ii) an award of common stock with a value of $40,000 each quarter for the first 8 calendar quarters following the first anniversary of the Advisor Agreement (totaling $160,000 annually). We issued 600,000 shares of restricted common stock that vest quarterly over one year in connection
99
with an amendment to the Advisor Agreement in July 2022. The Advisor Agreement may be terminated by either party without cause upon 180 days advance written notice. We may terminate the Advisor Agreement for material breach of the agreement if not cured after two weeks’ written notice. We will have no obligation to the advisor upon any termination of the agreement except for reimbursement of any unreimbursed expenses and pro-rata vesting of the equity awards issued under the agreement through the effective date of the termination.
Indemnification Agreements and Directors’ and Officers’ Liability Insurance
We have entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us to indemnify each director and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, penalties fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer.
Policies and Procedures for Related Party Transactions
Our board of directors has adopted a written related person transaction policy, to be effective upon the consummation of this offering, setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our audit committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction with an unrelated third party and the extent of the related person’s interest in the transaction. All of the transactions described in this section occurred prior to the adoption of this policy.
100
The following table sets forth information with respect to the beneficial ownership of our common stock as of September 30, 2022, and as adjusted to reflect the sale of our common stock offered by us in this offering, for:
• our named executive officer;
• each of our directors;
• all of our current directors and executive officers as a group; and
• each person, or group of affiliated persons, known by us to be the beneficial owner of more than 5% of our outstanding shares of common stock.
We have determined beneficial ownership in accordance with the rules of the SEC, which generally means that a person has beneficial ownership of a security if he or she possesses sole or shared voting or investment power of that security, including options that are currently exercisable or exercisable within 60 days of September 30, 2022. Unless otherwise indicated, to our knowledge, the persons and entities named in the table below have sole voting and sole investment power with respect to all shares that they beneficially own, subject to community property laws where applicable. The information in the table below does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Sections 13(d) and 13(g) of the Securities Act.
We have based our calculation of the percentage of beneficial ownership prior to this offering on 30,107,127 shares of our common stock outstanding as of September 30, 2022. We have based our calculation of the percentage of beneficial ownership after this offering on 31,607,127 shares of our common stock outstanding immediately after the closing of this offering. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that individual or entity, shares of common stock subject to options, convertible securities or other rights, held by such person that are currently exercisable or will become exercisable within 60 days of September 30, 2022, are considered outstanding. We did not, however, deem such shares outstanding for the purpose of computing the percentage ownership of any other individual or entity.
Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o 433 Plaza Real, Suite 275, Boca Raton, Florida 33432.
|
Name of Beneficial Owner |
Number of |
Percentage of Shares |
||||||
|
Before |
After |
|||||||
|
Greater than 5% Holders: |
|
|
||||||
|
Broadband Capital Investments LLC(1) |
1,590,000 |
5.3 |
% |
5.0 |
% |
|||
|
Einar Ronander, Ph.D.(2) |
3,597,424 |
11.9 |
% |
11.4 |
% |
|||
|
Executive Officers and Directors: |
|
|
|
|||||
|
Paul Mann(3) |
3,287,111 |
10.8 |
% |
10.3 |
% |
|||
|
Robert Ainscow(4) |
326,167 |
1.1 |
% |
1.0 |
% |
|||
|
Hendrick Strydom, Ph.D.(5) |
3,597,423 |
11.9 |
% |
11.4 |
% |
|||
|
Josh Donfeld(6) |
856,000 |
2.8 |
% |
2.7 |
% |
|||
|
Duncan Moore, Ph.D.(7) |
256,000 |
* |
|
* |
|
|||
|
Sergey Vasnetsov(8) |
2,200,000 |
7.3 |
% |
7.0 |
% |
|||
|
Todd Wider, M.D.(9) |
256,000 |
* |
|
* |
|
|||
|
All current executive officers and directors as a group (7 persons)(10) |
10,778,701 |
35.8 |
% |
34.1 |
% |
|||
____________
* Represents beneficial ownership of less than 1%.
(1) The address of Broadband Capital Investments LLC is 105 S Narcissus Avenue, Suite 705, West Palm Beach, FL 33401. Michael Rapoport serves as managing member of Broadband Capital Investments, LLC, and in such capacity has voting and dispositive power over the securities held by Broadband Capital Investments, LLC.
(2) Such shares are held by Carlein Investments (Pty) Ltd whose address is Building 46, CSIR Campus, Meiring Naude Road, Brummeria, Pretoria, 0184. Dr. Ronander has voting and dispositive power over such shares.
(3) Consists of (i) 1,550,000 shares of common stock held by Mr. Mann, (ii) 1,500,000 shares of performance-based restricted common stock granted by us to Mr. Mann in October 2021 and (iii) 237,111 shares of common stock issuable upon exercise of options held by Mr. Mann exercisable within 60 days of September 30, 2022.
101
(4) Consists of (i) 250,000 shares of common stock held by Mr. Ainscow and (ii) 76,167 shares of common stock issuable upon exercise of options held by Mr. Ainscow exercisable within 60 days of September 30, 2022.
(5) Such shares are held by Tianne Holdings (Pty) Ltd whose address is Building 46, CSIR Campus, Meiring Naude Road, Brummeria, Pretoria, 0184. Dr. Strydom has voting and dispositive power over such shares.
(6) Consists of 56,000 shares of common stock issuable upon exercise of options held by Mr. Donfeld exercisable within 60 days of September 30, 2022.
(7) Consists of 56,000 shares of common stock issuable upon exercise of options held by Dr. Moore exercisable within 60 days of September 30, 2022.
(8) 1,000,000 of such shares are held by Elista LLC (which is owned by Eliona Trust, a family trust, of which Mr. Vasnetsov is a trustee) whose address is P.O. Box 2291, Toa Baja 00951 Puerto Rico. Mr. Vasnetsov has voting and dispositive power over such shares as trustee. 600,000 of such shares, which are restricted stock that vest annually over three years and are subject to forfeiture, and 600,000 of such shares, which are restricted stock that vest over one year and are subject to forfeiture, are held by ChemBridges LLC whose address is P.O. Box 2291, Toa Baja 00951 Puerto Rico. Mr. Vasnetsov has voting and dispositive power over such shares as the President and owner of ChemBridges LLC.
(9) Consists of 56,000 shares of common stock issuable upon exercise of options held by Dr. Wider exercisable within 60 days of September 30, 2022.
(10) Includes the shares described in notes 3, 4, 5, 6, 7 and 8 above.
102
Selling Stockholder Shares
This prospectus includes the possible resale by the selling stockholders identified in the table below of up to 2,057,500 shares of our common stock. The selling stockholder shares were originally issued and sold to purchasers in private placement transactions in late November 2021 through April 2022 at a purchase price of $2.00 per share.
The selling stockholders may sell some, all or none of their selling stockholder shares. We do not know how long the selling stockholders will hold the selling stockholder shares before selling them, and we currently have no agreements, arrangements or understandings with the selling stockholders regarding the sale of any of the selling stockholder shares. Unless otherwise indicated in the footnotes to the below table, no selling stockholder has had any material relationship with us or any of our affiliates within the past three years other than as a securityholder of our company.
We have prepared the following table based on written representations and information furnished to us by or on behalf of the selling stockholders. Since the date on which the selling stockholders provided this information, the selling stockholders may have sold, transferred or otherwise disposed of all or a portion of the shares in a transaction exempt from the registration requirements of the Securities Act. Unless otherwise indicated in the footnotes below, we believe that: (i) none of the selling stockholders are broker-dealers or affiliates of broker-dealers, and (ii) no selling stockholder has direct or indirect agreements or understandings with any person to distribute their selling stockholder shares. To the extent any selling stockholder identified below is, or is affiliated with, a broker-dealer, it could be deemed to be an “underwriter” within the meaning of the Securities Act. Information about the selling stockholders may change over time.
The following table presents information regarding the selling stockholders and the selling stockholder shares that each may offer and sell from time to time under this prospectus. The table is prepared based on information supplied to us by the selling stockholders, and reflects their respective holdings as of September 30, 2022, unless otherwise noted in the footnotes to the table. Beneficial ownership is determined in accordance with the rules of the SEC, and thus represents voting or investment power with respect to our securities. Under such rules, beneficial ownership includes any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has the right to acquire within 60 days after the date of this table. To our knowledge and subject to applicable community property rules, the persons and entities named in the table have sole voting and sole investment power with respect to all equity interests beneficially owned. The percentage of shares beneficially owned before and after the offering is based on 30,107,127 shares of our common stock issued and outstanding on September 30, 2022, and 31,607,127 shares issued and outstanding after the offering.
|
Selling Stockholder |
Number of |
Number of |
Number of |
Percentage |
||||
|
Aikido Labs, LLC(2) |
500,000 |
500,000 |
— |
— |
||||
|
Alex Kramer |
5,000 |
5,000 |
— |
— |
||||
|
Andreas Typaldos Family Limited Partnership(3) |
50,000 |
50,000 |
— |
— |
||||
|
Anthony and Cynthia Mancuso |
5,000 |
5,000 |
— |
— |
||||
|
Atwater Consulting LLC(4) |
50,000 |
50,000 |
— |
— |
||||
|
Beverly Vorhees Foley Revocable Trust(5) |
50,000 |
50,000 |
— |
— |
||||
|
Brian Rhoads |
25,000 |
25,000 |
— |
— |
||||
|
Christopher Holme |
5,000 |
5,000 |
— |
— |
||||
|
Christos Morris |
12,500 |
12,500 |
— |
— |
||||
|
Crystalia Investments Ltd.(6) |
50,000 |
50,000 |
— |
— |
||||
|
David Ohl |
25,000 |
25,000 |
— |
— |
103
|
Selling Stockholder |
Number of |
Number of |
Number of |
Percentage |
||||
|
Dragon Dynamic Catalytic Bridge SAC Fund(7) |
125,000 |
125,000 |
— |
— |
||||
|
Eleven Ventures LLC(8) |
250,000 |
250,000 |
— |
— |
||||
|
Gustavo Perez |
50,000 |
50,000 |
— |
— |
||||
|
Jaime Enrique Y. Gonzalez |
12,500 |
12,500 |
— |
— |
||||
|
Jennifer Wool Cottone |
10,000 |
10,000 |
— |
— |
||||
|
Jinho Yu |
5,000 |
5,000 |
— |
— |
||||
|
Josh Shipley |
5,000 |
5,000 |
— |
— |
||||
|
Joseph Smith |
25,000 |
25,000 |
||||||
|
Joshua Lipman |
25,000 |
25,000 |
||||||
|
Justin Boyle |
100,000 |
100,000 |
— |
— |
||||
|
Kenneth C Greenberg |
12,500 |
12,500 |
— |
— |
||||
|
Lewis Smith |
25,000 |
25,000 |
— |
— |
||||
|
MMRB LLC(9) |
5,000 |
5,000 |
— |
— |
||||
|
Mohsen Khorassani |
25,000 |
25,000 |
— |
— |
||||
|
Paul Prasarn |
2,500 |
2,500 |
— |
— |
||||
|
Quarto Capital Investment Ltd(10) |
50,000 |
50,000 |
— |
— |
||||
|
Simon Becker |
50,000 |
50,000 |
— |
— |
||||
|
Stephen Chruscicki |
2500 |
2500 |
— |
— |
||||
|
Steve Scopellite |
50,000 |
50,000 |
— |
— |
||||
|
Steve Scopellite 2021 Irrevocable Trust(11) |
125,000 |
125,000 |
— |
— |
||||
|
Steven Deluca |
50,000 |
50,000 |
— |
— |
||||
|
The Catherine Foley Carlson Trust(12) |
50,000 |
50,000 |
— |
— |
||||
|
The Spencer Martin Foley Trust(13) |
100,000 |
100,000 |
— |
— |
||||
|
Thomas Garcia |
17,500 |
17,500 |
— |
— |
||||
|
Tim Buckley |
7,500 |
7,500 |
— |
— |
||||
|
William Ciccarelli |
25,000 |
25,000 |
— |
— |
||||
|
Xiao Fu |
25,000 |
25,000 |
— |
— |
||||
|
Xin Wang |
50,000 |
50,000 |
— |
— |
||||
|
Total |
2,057,500 |
2,057,500 |
— |
— |
____________
* Represents beneficial ownership of less than 1%.
(1) Assumes all shares offered by the selling stockholders hereby are sold and that the selling stockholders buy or sell no additional shares of common stock prior to the completion of this offering. The registration of these shares does not necessarily mean that the selling stockholders will sell all or any portion of the shares covered by this prospectus.
(2) Aikido Labs, LLC is controlled by its board of directors.
(3) Andreas Typaldos Family Limited Partnership is controlled by Andreas Typaldos.
(4) Atwater Consulting LLC is controlled by Anthony Hayes.
(5) Beverly Vorhees Foley Revocable Trust is controlled by Beverly V. Foley.
(6) Crystalia Investments Ltd. is controlled by Wendy Yap.
(7) Dragon Dynamic Catalytic Bridge SAC Fund is controlled by James Michie and Gary Carr, as directors.
(8) Eleven Ventures LLC is controlled by Ian McKenzie.
(9) MMRB LLC is controlled by Murphy Bright.
(10) Quarto Capital Investment Ltd is controlled by George Djuhari.
(11) Steve Scopellite 2021 Irrevocable Trust is controlled by Michael Canarick.
(12) The Catherine Foley Carlson Trust is controlled by Catherine Foley Carlson.
(13) The Spencer Martin Foley Trust is controlled by Spencer Foley.
104
Plan of Distribution
We are registering the selling stockholder shares to permit the resale of the selling stockholder shares by the selling stockholders from time to time after the date of this prospectus. We will not receive any of the proceeds from the sale of the selling stockholder shares. We will bear all fees and expenses incident to the registration of the selling stockholder shares in the registration statement of which this prospectus forms a part. The selling stockholder shares will not be sold through Revere Securities, LLC in this initial public offering.
The selling stockholders may sell all or a portion of the selling stockholder shares beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the selling stockholder shares are sold through underwriters or broker-dealers, the selling stockholders will be responsible for underwriting discounts or commissions or agent’s commissions. The selling stockholder shares may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions,
• on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale;
• in the over-the-counter market;
• in transactions otherwise than on these exchanges or systems or in the over-the-counter market;
• ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
• block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction;
• purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
• an exchange distribution in accordance with the rules of the applicable exchange;
• privately negotiated transactions;
• short sales;
• in transactions through broker-dealers that agree with the selling stockholders to sell a specified number of such securities at a stipulated price per security;
• through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
• a combination of any such methods of sale; or
• any other method permitted pursuant to applicable law.
The selling stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act, if available, rather than under this prospectus. However, the selling stockholders will not sell any selling stockholder shares until after the closing of this offering.
If the selling stockholders effect such transactions by selling shares to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholders or commissions from purchasers of the selling stockholder shares for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved). In connection with sales of the selling stockholder shares or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the selling stockholder shares in the course of hedging in positions they assume. The selling stockholders may also sell selling stockholder shares short and deliver selling stockholder shares covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling stockholders may also loan or pledge selling stockholder shares to broker-dealers that in turn may sell such shares.
105
The selling stockholders may pledge or grant a security interest in some or all of the warrants or selling stockholder shares owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the selling stockholder shares from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act, amending, if necessary, the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate the selling stockholder shares in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The selling stockholders and any broker-dealer participating in the distribution of the selling stockholder shares may be deemed to be “underwriters” within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the selling stockholder shares is made, a prospectus supplement, if required, will be distributed which will set forth the aggregate amount of selling stockholder shares being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling stockholders and any discounts, commissions or concessions allowed or reallowed or paid to broker-dealers.
Under the securities laws of some states, the selling stockholder shares may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the selling stockholder shares may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any selling stockholder will sell any or all of the selling stockholder shares registered pursuant to the registration statement, of which this prospectus forms a part.
The selling stockholders and any other person participating in such distribution will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including, without limitation, Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the selling stockholder shares by the selling stockholders and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of the selling stockholder shares to engage in market-making activities with respect to the selling stockholder shares. All of the foregoing may affect the marketability of the selling stockholder shares and the ability of any person or entity to engage in market-making activities with respect to the selling stockholder shares.
Once sold under the registration statement, of which this prospectus forms a part, the selling stockholder shares will be freely tradeable in the hands of persons other than our affiliates.
106
General
As of the closing of this offering, our authorized capital stock will consist of 500,000,000 shares of common stock, par value $0.01 per share, and 10,000,000 shares of preferred stock, par value $0.01 per share.
The following descriptions of our capital stock and provisions of our Certificate of Incorporation and our Bylaws are summaries and are qualified by reference to the full text of those documents, copies of which will be filed with the SEC as exhibits to the registration statement of which this prospectus forms a part. The following summary of relevant provisions of the DGCL is qualified by the full text of such provisions. The description of our capital stock reflects changes to our capital structure that will occur prior to the closing of this offering.
Because these are only summaries, they do not contain all the information that may be important to you. We expect to adopt a restated certificate of incorporation and restated bylaws that will become effective upon the completion of this offering, and this description summarizes provisions that are expected to be included in these documents.
Common Stock
Outstanding Shares
As of September 30, 2022, we had 30,107,127 shares of common stock outstanding, held of record by 106 stockholders.
Dividend Rights
Subject to preferences that may apply to any shares of preferred stock outstanding at the time, the holders of our common stock will be entitled to receive dividends out of funds legally available if our board of directors, in its discretion, determines to issue dividends and then only at the times and in the amounts that our board of directors may determine.
Voting Rights
Holders of common stock will be entitled to one vote for each share on all matters submitted to a vote of stockholders.
Our Certificate of Incorporation will not provide for cumulative voting for the election of directors. Our Certificate of Incorporation and Bylaws will establish a classified board of directors that is divided into three classes with staggered three-year terms. Only the directors in one class will be subject to election by a plurality of the votes cast at each annual meeting of our stockholders, with the directors in the other classes continuing for the remainder of their respective three-year terms.
No Preemptive or Similar Rights
Our common stock will not be entitled to preemptive rights, and is not subject to conversion, redemption or sinking fund provisions.
Right to Receive Liquidation Distributions
If we become subject to a liquidation, dissolution or winding-up, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our common stock and any participating preferred stock outstanding at that time, subject to prior satisfaction of all outstanding debt and liabilities and the preferential rights of and the payment of liquidation preferences, if any, on any outstanding shares of preferred stock.
Fully Paid and Non-Assessable
All of the outstanding shares of our common stock are, and the shares of our common stock to be issued pursuant to this offering will be, fully paid and non-assessable.
107
Preferred Stock
No shares of preferred stock will be issued or outstanding immediately after the offering contemplated by this prospectus. Our Certificate of Incorporation will authorize our board of directors to establish one or more series of preferred stock (including convertible preferred stock). Unless required by law or any stock exchange, the authorized shares of preferred stock will be available for issuance without further action by the holders of our common stock. Our board of directors will be able to determine, with respect to any series of preferred stock, the powers (including voting powers), preferences and relative, participating, optional or other special rights, and the qualifications, limitations, or restrictions thereof, including:
• the designation of the series;
• the number of shares of the series, which our board of directors may, except where otherwise provided in the preferred stock designation, increase (but not above the total number of authorized shares of the class) or decrease (but not below the number of shares then outstanding);
• whether dividends, if any, will be cumulative or non-cumulative and the dividend rate of the series;
• the dates at which dividends, if any, will be payable;
• the redemption or repurchase rights and price or prices, if any, for shares of the series;
• the terms and amounts of any sinking fund provided for the purchase or redemption of shares of the series;
• the amounts payable on shares of the series in the event of any voluntary or involuntary liquidation, dissolution or winding-up of our affairs;
• whether the shares of the series will be convertible into shares of any other class or series, or any other security, of us or any other entity, and, if so, the specification of the other class or series or other security, the conversion price or prices or rate or rates, any rate adjustments, the date or dates as of which the shares will be convertible and all other terms and conditions upon which the conversion may be made;
• restrictions on the issuance of shares of the same series or of any other class or series; and
• the voting rights, if any, of the holders of the series.
We could issue a series of preferred stock that could, depending on the terms of the series, impede or discourage an acquisition attempt or other transaction that some, or a majority, of the holders of our common stock might believe to be in their best interests or in which the holders of our common stock might receive a premium over the market price of the shares of our common stock. Additionally, the issuance of preferred stock may adversely affect the rights of holders of our common stock by restricting dividends on the common stock, diluting the voting power of the common stock, or subordinating the liquidation rights of the common stock. As a result of these or other factors, the issuance of preferred stock could have an adverse impact on the market price of our common stock.
Registration Rights
Our subscription agreements with certain investors provides certain holders the right, following the date of this prospectus, to request that their shares be included in a registration statement that we are otherwise filing.
Anti-Takeover Matters in our Governing Documents and Under Delaware Law
Our Certificate of Incorporation and our Bylaws will contain, and the DGCL contains, provisions that are intended to enhance the likelihood of continuity and stability in the composition of our board of directors. These provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile or abusive change of control, and enhance the ability of our board of directors to maximize stockholder value in connection with any unsolicited offer to acquire us. However, these provisions may have an antitakeover effect and may delay, deter, or prevent a merger or acquisition by means of a tender offer, a proxy contest, or other takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the prevailing market price for the shares of common stock held by stockholders.
108
Authorized but unissued capital stock
The authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval, subject to any limitations imposed by the listing standards of Nasdaq. These additional shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could make more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger, or otherwise.
Classified board of directors
Our Certificate of Incorporation will provide that our board of directors will be divided into three classes, with the classes as nearly equal in number as possible and each class serving three-year staggered terms. Directors may only be removed from our board of directors for cause by the affirmative vote of at least 66²⁄₃ of the voting power of all of our then-outstanding shares of capital stock entitled to vote generally in the election of directors, voting together as a single class. In addition, our Certificate of Incorporation will provide that, subject to the rights granted to one or more series of preferred stock then outstanding, any newly created directorship on the board of directors that results from an increase in the number of directors and any vacancies on our board of directors will be filled only by the affirmative vote of a majority of the remaining directors, even if less than a quorum, or by a sole remaining director. After this offering, a director chosen to fill a position resulting from an increase in the number of directors will hold office until the next election of the director’s class and until the director’s successor is duly elected and qualified, or until the director’s earlier death, resignation or removal. These provisions may have the effect of deferring, delaying, or discouraging hostile takeovers, changes in control of us or changes in our management.
Delaware Anti-Takeover Law
After this offering, we will be subject to Section 203 of the DGCL, which is an anti-takeover law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder for a period of three years following the date that the person became an interested stockholder, unless the business combination or the transaction in which the person became an interested stockholder is approved in a prescribed manner. Generally, a business combination includes a merger, asset or stock sale, or another transaction resulting in a financial benefit to the interested stockholder. Generally, an interested stockholder is a person who, together with affiliates and associates, owns 15% or more of the corporation’s outstanding voting stock or is the corporation’s affiliate or associate and was the owner of 15% or more of the corporation’s outstanding voting stock at any time within the three-year period immediately before the date of determination. The existence of this provision may have an anti-takeover effect with respect to transactions that are not approved in advance by our board, including discouraging attempts that might result in a premium over the market price for the shares of common stock held by stockholders.
No cumulative voting
Under Delaware law, the right to vote cumulatively does not exist unless the certificate of incorporation specifically authorizes cumulative voting. Our Certificate of Incorporation will not authorize cumulative voting. Therefore, stockholders holding a majority of the shares of our stock entitled to vote generally in the election of directors will be able to elect all of our directors.
Special stockholder meetings
Our Certificate of Incorporation will provide that special meetings of our stockholders may be called at any time only by or at the direction of the board of directors, the chair of the board of directors or our Chief Executive Officer. Our Bylaws will prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions may have the effect of deferring, delaying, or discouraging hostile takeovers or changes in control or management.
Director nominations and stockholder proposals
Our Bylaws will establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors or a committee of the board of directors. In order for any matter to be “properly brought” before a meeting, a
109
stockholder will have to comply with advance notice requirements and provide us with certain information. Generally, to be timely, a stockholder’s notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary date of the immediately preceding annual meeting of stockholders. Our Bylaws will also specify requirements as to the form and content of a stockholder’s notice. Our Bylaws will allow the chair of a meeting of the stockholders to adopt rules and regulations for the conduct of that meeting that may have the effect of precluding the conduct of certain business at that meeting if the rules and regulations are not followed. These provisions may also defer, delay, or discourage a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to influence or obtain control.
Stockholder action by written consent
Pursuant to Section 228 of the DGCL, any action required to be taken at any annual or special meeting of the stockholders may be taken without a meeting, without prior notice, and without a vote if a consent or consents in writing, setting forth the action so taken, is or are signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares of stock entitled to vote thereon were present and voted, unless the certificate of incorporation provides otherwise. Our Certificate of Incorporation will preclude stockholder action by written consent, subject to the rights of the holders of any series of preferred stock.
Amendment of Certificate of Incorporation or Bylaws
The DGCL provides generally that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s certificate of incorporation or bylaws, unless a corporation’s certificate of incorporation or bylaws, as the case may be, requires a greater percentage. Upon the closing of this offering, our Bylaws may be amended or repealed by a majority vote of our board of directors or by the affirmative vote of the holders of at least 662⁄3% of the votes which all our stockholders would be entitled to cast in any annual election of directors. In addition, the affirmative vote of the holders of at least 662⁄3% of the votes which all our stockholders would be entitled to cast in any election of directors will be required to amend or repeal or to adopt any provisions inconsistent with any of the provisions of our Certificate of Incorporation described above.
The foregoing provisions of our Certificate of Incorporation and our Bylaws could discourage potential acquisition proposals and could delay or prevent a change in control. These provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in the policies formulated by our board of directors and to discourage certain types of transactions that may involve an actual or threatened change of control. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our shares of common stock that could result from actual or rumored takeover attempts. Such provisions also may have the effect of preventing changes in our management or delaying or preventing a transaction that might benefit you or other minority stockholders.
Exclusive forum
Our Certificate of Incorporation will provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware be the sole and exclusive forum for: (1) any derivative action or proceeding brought on behalf of our company, (2) any action asserting a claim of breach of fiduciary duty owed by any director, officer, agent, or other employee or stockholder of our company to us or our stockholders, (3) any action asserting a claim arising pursuant to any provision of the DGCL, our Certificate of Incorporation or our Bylaws or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware, or (4) any action asserting a claim governed by the internal affairs doctrine, in each case subject to such Court of Chancery having personal jurisdiction over the indispensable parties named as defendants therein. It will further provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall, to the fullest extent permitted by law, be the sole and exclusive forum for the resolutions of any complaint asserting a cause of action arising under the Securities Act. The exclusive forum clauses described above shall not apply to suits
110
brought to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Although we believe these provisions benefit us by providing increased consistency in the application of applicable law in the types of lawsuits to which they apply, the provisions may have the effect of discouraging lawsuits against our directors and officers. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings and there is uncertainty as to whether a court would enforce such provisions. In addition, investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. It is possible that, in connection with any applicable action brought against us, a court could find the choice of forum provisions contained in our Certificate of Incorporation to be inapplicable or unenforceable in such action. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and consented to the forum provisions in our Certificate of Incorporation.
Limitations of liability and indemnification
The DGCL authorizes corporations to limit or eliminate the personal liability of directors to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. Our Certificate of Incorporation includes a provision that eliminates the personal liability of directors for monetary damages to the corporation or its stockholders for any breach of fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL. The effect of these provisions is to eliminate the rights of us and our stockholders, through stockholders’ derivative suits on our behalf, to recover monetary damages from a director for breach of fiduciary duty as a director, including breaches resulting from grossly negligent behavior. However, exculpation does not apply to any breaches of the director’s duty of loyalty, any acts or omissions not in good faith or that involve intentional misconduct or knowing violation of law, any authorization of dividends or stock redemptions or repurchases paid or made in violation of the DGCL, or for any transaction from which the director derived an improper personal benefit.
Our Bylaws will generally provide that we must indemnify and advance expenses to our directors and officers to the fullest extent authorized by the DGCL. We also are expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for our directors, officers and certain employees for some liabilities. We believe these indemnification and advancement provisions and insurance are useful to attract and retain qualified directors and executive officers.
The limitation of liability, indemnification and advancement provisions in our Certificate of Incorporation and our Bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. In addition, your investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
There is currently no pending material litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought.
We intend to enter into an indemnification agreement with each of our directors and executive officers as described in “Certain Relationships and Related Party Transactions — Indemnification agreements.” Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors or executive officers, we have been informed that in the opinion of the SEC such indemnification is against public policy and is therefore unenforceable.
Transfer Agent and Registrar
Upon the closing of this offering, the transfer agent and registrar for our common stock will be American Stock Transfer & Trust Company, LLC. The transfer agent’s address is 6201 15th Avenue, Brooklyn, New York 11219.
Nasdaq Listing
We have applied to list our common stock on the Nasdaq Capital Market under the symbol “ASPI.”
111
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock, and we cannot predict the effect, if any, that market sales of shares of our common stock or the availability of shares of our common stock for sale will have on the market price of our common stock prevailing from time to time. Future sales of our common stock in the public market, or the availability of such shares for sale in the public market, could adversely affect market prices prevailing from time to time. As described below, only a limited number of shares will be available for sale shortly after this offering due to contractual and legal restrictions on resale. Nevertheless, sales of our common stock in the public market after such restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price at such time and our ability to raise equity capital in the future.
Following the completion of this offering, based on the number of shares of our capital stock outstanding as of September 30, 2022, we will have a total of 31,607,127 shares of our common stock. Of these outstanding shares, all of the 1,500,000 shares of common stock sold in this offering will be freely tradable, except that any shares purchased in this offering by our affiliates, as that term is defined in Rule 144 under the Securities Act, would only be able to be sold in compliance with the Rule 144 limitations described below.
The remaining outstanding shares of our common stock will be deemed “restricted securities” as defined in Rule 144. Restricted securities may be sold in the public market only if they are registered or if they qualify for an exemption from registration under Rule 144 or Rule 701 under the Securities Act, which rules are summarized below. In addition, all of our executive officers, directors and holders of approximately 90% of our common stock and securities convertible into or exchangeable for our common stock have entered into market standoff agreements with us or lock-up agreements with the underwriters under which they have agreed, subject to specific exceptions, not to sell any of our stock for at least 180 days following the date of this prospectus. As a result of these agreements, subject to the provisions of Rule 144 or Rule 701, shares will be available for sale in the public market as follows:
• beginning on the date of this prospectus, the 1,500,000 shares of common stock sold in this offering will be immediately available for sale in the public market;
• beginning 181 days after the date of this prospectus, subject to certain exceptions as described in the section titled “Underwriting” below, 28,711,294 additional shares of common stock will become eligible for sale in the public market, of which 14,376,125 shares will be held by affiliates and subject to the volume and other restrictions of Rule 144, as described below; and
• the remainder of the shares of common stock will be eligible for sale in the public market from time to time thereafter, subject in some cases to the volume and other restrictions of Rule 144, as described below.
Lock-Up Agreements
We, our officers, directors and holders of approximately 90% of our capital stock and securities convertible into or exchangeable for our capital stock have agreed or will agree, with the underwriters, that, subject to certain exceptions, for a period of 180 days after the date of this prospectus, we and they will not, and will not cause or direct any of our or their respective affiliates to, without the prior written consent of Revere Securities, LLC, (i) offer, sell, contract to sell, pledge, grant any option to purchase, lend or otherwise dispose of any shares of common stock, any options or warrants to purchase any shares of common stock or any securities convertible into or exchangeable for or that represent the right to receive shares of our common stock, (ii) engage in any hedging or other transaction or arrangement (including, without limitation, any short sale or the purchase or sale of, or entry into, any put or call option, or combination thereof, forward, swap or any other derivative transaction or instrument, however described or defined) which is designed to or which reasonably could be expected to lead to or result in a sale, loan, pledge or other disposition (whether by such holder or someone other than such holder), or transfer of any of the economic consequences of ownership, in whole or in part, directly or indirectly, of any shares of common stock or derivative instruments, whether any such transaction or arrangement (or instrument provided for thereunder) would be settled by delivery of common stock or other securities, in cash or otherwise, or (iii) otherwise publicly announce any intention to engage in or cause any action or activity described in clauses (i) or (ii) above. Revere Securities, LLC may, in their discretion, release any of the securities subject to lock-up agreements at any time. When determining whether or not to release our common stock and other securities from lock-up agreements, Revere Securities, LLC will consider, among other factors, the holder’s reasons for requesting the release, the
112
number of shares for which the release is being requested and market conditions at the time of the request. In the event of such a release or waiver for one of our directors or officers, Revere Securities, LLC shall provide us with notice of the impending release or waiver at least three business days before the effective date of such release or waiver and we will announce the impending release or waiver by issuing a press release at least two business days before the effective date of the release or waiver.
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to the public company reporting requirements of Section 13 or Section 15(d) of the Exchange Act for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares of our common stock proposed to be sold for at least six months is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person would be entitled to sell those shares without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, our affiliates or persons selling shares of our common stock on behalf of our affiliates are entitled to sell upon expiration of the market standoff agreements and lock-up agreements described above, within any three-month period, a number of shares that does not exceed the greater of:
• 1% of the number of shares of our capital stock then outstanding, which will equal 316,071 shares immediately after this offering; or
• the average weekly trading volume of our common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale.
• Sales under Rule 144 by our affiliates or persons selling shares of our common stock on behalf of our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our capital stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling those shares pursuant to Rule 701.
Registration Statement
We intend to file a registration statement on Form S-8 under the Securities Act promptly after the closing of this offering to register shares of our common stock issued or reserved for issuance under our 2022 Plan. The registration statement on Form S-8 is expected to become effective immediately upon filing, and shares of our common stock covered by the registration statement will then become eligible for sale in the public market, subject to the Rule 144 limitations applicable to affiliates, vesting restrictions and any applicable market standoff agreements and lock-up agreements. See the section captioned “Executive Compensation — Employee Benefit and Equity Incentive Plans” for a description of our equity compensation plans.
113
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS TO NON-U.S. HOLDERS
The following is a summary of the material U.S. federal income tax considerations to non-U.S. holders (as defined below) of the ownership and disposition of our common stock issued pursuant to this offering. This discussion is not a complete analysis of all potential U.S federal income tax considerations relating thereto, does not address the potential application of the Medicare contribution tax on net investment income, and does not address any U.S. estate or gift tax consequences, generation-skipping tax, the excise tax on stock repurchases, or any tax consequences arising under any state, local or foreign tax laws, or any other U.S. federal tax laws. This discussion is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury regulations promulgated thereunder (“Treasury Regulations”), judicial decisions and published rulings and administrative pronouncements of the IRS, all as in effect on the date of this prospectus supplement. Any of the authorities on which this summary is based could be changed in a material and adverse manner possibly with retroactive effect, at any time. We have not requested a ruling from the Internal Revenue Service (“IRS”) with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS or a court will agree with such statements and conclusions.
This discussion is limited to non-U.S. holders who hold our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all of the U.S. federal income tax consequences that may be relevant to an individual holder in light of such holder’s particular circumstances. This discussion also does not consider any specific facts or circumstances that may be relevant to non-U.S. holders subject to special rules under the U.S. federal income tax laws, including:
• certain former citizens or long-term residents of the United States;
• partnerships or other pass-through entities (and investors therein);
• “controlled foreign corporations”;
• “passive foreign investment companies”;
• corporations that accumulate earnings to avoid U.S. federal income tax;
• banks, financial institutions, investment funds, insurance companies, brokers, dealers or traders in securities;
• tax-exempt organizations and governmental organizations;
• tax-qualified retirement plans;
• persons subject to the alternative minimum tax;
• persons subject to special tax accounting rules under Section 451(b) of the Code;
• persons that own or have owned, actually or constructively, more than 5% of our common stock;
• persons who have elected to mark securities to market; and
• persons holding our common stock as part of a hedging or conversion transaction or straddle, or a constructive sale, or other risk reduction strategy or integrated investment.
PROSPECTIVE INVESTORS SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES TO THEM OF ACQUIRING, OWNING AND DISPOSING OF OUR COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL OR FOREIGN TAX LAWS AND ANY OTHER U.S. FEDERAL TAX LAWS.
114
Definition of Non-U.S. Holder
For purposes of this discussion, a non-U.S. holder is any beneficial owner of our common stock that is not a “U.S. person” or a partnership (including any entity or arrangement treated as a partnership) or other pass-through entity for U.S. federal income tax purposes. A U.S. person is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
• an individual who is a citizen or resident of the United States;
• a corporation (including any entity treated as a corporation for U.S. federal income tax purposes) created or organized under the laws of the United States, any state thereof or the District of Columbia;
• an estate, the income of which is subject to U.S. federal income tax regardless of its source; or
• a trust (1) whose administration is subject to the primary supervision of a U.S. court and which has one or more U.S. persons who have the authority to control all substantial decisions of the trust or (2) that has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person for U.S. federal income tax purposes.
For purposes of this discussion, a non-U.S. holder does not include a partnership (including for this purpose any entity that is treated as a partnership or other pass-through entity for U.S. federal income tax purposes). If a partnership or other pass-through entity is a beneficial owner of our common stock, the tax treatment of a partner or other owner will generally depend upon the status of the partner (or other owner) and the activities of the entity. If you are a partner (or other owner) of a pass-through entity that acquires our common stock, you should consult your tax advisor regarding the tax considerations of acquiring, owning and disposing of our common stock. Also, it is important to note that the rules for determining whether an individual is a non-resident alien for income tax purposes differ from those applicable for estate tax purposes.
If you are an individual non-U.S. citizen, you may, in some cases, be deemed to be a resident alien (as opposed to a nonresident alien) by virtue of being present in the United States for at least 31 days in the calendar year and for an aggregate of at least 183 days during a three-year period ending in the current calendar year. Generally, for this purpose, all the days present in the current year, one-third of the days present in the immediately preceding year, and one-sixth of the days present in the second preceding year, are counted.
Resident aliens are generally subject to U.S. federal income tax as if they were U.S. citizens. Individuals who are uncertain of their status as resident or nonresident aliens for U.S. federal income tax purposes are urged to consult their own tax advisors regarding the U.S. federal income tax consequences of the ownership or disposition of our common stock.
Distributions on Our Common Stock
If we distribute cash or other property on our common stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts distributed in excess of our current and accumulated earnings and profits will constitute a return of capital and will first be applied against and reduce a non-U.S. holder’s tax basis in our common stock, but not below zero. Any distribution in excess of a non-U.S. holder’s basis will be treated as gain realized on the sale or other disposition of our common stock and will be treated as described in the “Gain on Disposition of Our Common Stock” section below.
Subject to the discussion below regarding effectively connected income, backup withholding and FATCA (as defined below), dividends paid to a non-U.S. holder of our common stock generally will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the distribution in which case the non-U.S. holder would be entitled to a refund from the IRS for the withholding tax on the portion of the distribution that exceeded our current and accumulated earnings and profits. In order to obtain reduced rate of U.S. federal withholding tax under an applicable income tax treaty, a non-U.S. holder will be required to furnish the applicable withholding agent with a valid IRS Form W-8BEN or IRS Form W-8BEN-E (or other applicable documentation) certifying such non-U.S. holder’s qualification for the reduced rate. This certification must be provided to the applicable withholding agent before the payment of dividends and must be updated periodically. If the non-U.S. holder holds our common
115
stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required to provide appropriate documentation to the agent, which then will be required to provide certification to the applicable withholding agent, either directly or through other intermediaries.
If a non-U.S. holder holds our common stock in connection with the conduct of a trade or business in the United States, and dividends paid on our common stock are effectively connected with such holder’s U.S. trade or business (and are attributable to such holder’s permanent establishment or fixed base in the United States if required by an applicable tax treaty), the non-U.S. holder will generally be exempt from U.S. federal withholding tax, provided that the non-U.S. holder furnishes a valid IRS Form W-8ECI (or applicable successor form) to the applicable withholding agent.
However, any such effectively connected dividends paid on our common stock generally will be subject to U.S. federal income tax on a net income basis at the regular U.S. federal income tax rates in the same manner as if such holder were a resident of the United States. A non-U.S. holder that is a foreign corporation also may be subject to an additional branch profits tax equal to 30% (or such lower rate specified by an applicable income tax treaty) of its effectively connected earnings and profits for the taxable year, as adjusted for certain items.
Non-U.S. holders that do not provide the required certification on a timely basis, but that qualify for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS. Non-U.S. holders should consult their tax advisors regarding any applicable income tax treaties that may provide for different rules.
Gain on Disposition of Our Common Stock
Subject to the discussion below regarding backup withholding tax and FATCA, a non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized on the sale or other disposition of our common stock, unless:
• the gain is effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the United States;
• the non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the disposition, and certain other requirements are met; or
• our common stock constitutes a “U.S. real property interest” by reason of our status as a “U.S. real property holding corporation” (“USRPHC”), for U.S. federal income tax purposes at any time within the shorter of the five-year period preceding the disposition or the non-U.S. holder’s holding period for our common stock, and our common stock is not regularly traded on an established securities market during the calendar year in which the sale or other disposition occurs.
Determining whether we are a USRPHC depends on the fair market value of our U.S. real property interests relative to the fair market value of our other trade or business assets and our foreign real property interests. In general, a corporation is a USRPHC if the fair market value of its “U.S. real property interests” equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests and its other assets used or held for use in a trade or business. In the event that we are determined to be a USRPHC, gain will not be subject to tax as U.S. trade or business income under Section 897 of the Code if a non-U.S. holder’s holdings (direct and indirect) at all times during the applicable period constituted 5% or less of our common stock, provided that our common stock was regularly traded on an established securities market during such period. We believe we are not currently and we do not anticipate becoming a USRPHC for U.S. federal income tax purposes. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property interests relative to the fair market value of our other business assets, there can be no assurance that we are not a USRPHC or will not become one in the future. Prospective investors are encouraged to consult their own tax advisors regarding the possible tax consequences to them if we are, or were to become, a USRPHC.
Gain described in the first bullet point above generally will be subject to U.S. federal income tax on a net income basis at the regular U.S. federal income tax rates in the same manner as if such non-U.S. holder were a resident of the United States. A non-U.S. holder that is a foreign corporation also may be subject to an additional branch profits tax equal to 30% (or such lower rate specified by an applicable income tax treaty) of its effectively connected
116
earnings and profits for the taxable year, as adjusted for certain items. Gain described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty), but may be offset by certain U.S.-source capital losses (even though the individual is not considered a resident of the United States), provided that the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses. Gain described in the third bullet point above will generally be subject to U.S. federal income tax in the same manner as gain that is effectively connected with the conduct of a U.S. trade or business (subject to any provisions under an applicable income tax treaty), except that the branch profits tax generally will not apply.
Non-U.S. holders should consult their tax advisors regarding any applicable income tax treaties that may provide for different rules.
Information Reporting and Backup Withholding
Annual reports are required to be filed with the IRS and provided to each non-U.S. holder indicating the amount of dividends on our common stock paid to such holder and the amount of any tax withheld with respect to those dividends. These information reporting requirements apply even if no withholding was required because the dividends were effectively connected with the holder’s conduct of a U.S. trade or business, or withholding was reduced or eliminated by an applicable income tax treaty. This information also may be made available under a specific treaty or agreement with the tax authorities in the country in which the non-U.S. holder resides or is established.
Backup withholding, currently at a 24% rate, generally will not apply to payments to a non-U.S. holder of dividends on or the gross proceeds of a disposition of our common stock provided the non-U.S. holder furnishes the required certification for its non-U.S. status, such as by providing a valid IRS Form W-8BEN, IRS Form W-8BEN-E or IRS Form W-8ECI, or certain other requirements are met.
Backup withholding is not an additional tax. If backup withholding results in an overpayment of taxes, a refund may be obtained, provided that the required information is timely furnished to the IRS. If any amount is withheld under the backup withholding rules, the non-U.S. holder should consult with a U.S. tax advisor regarding the possibility of and procedure for obtaining a refund or a credit against the non-U.S. holder’s U.S. federal income tax liability, if any.
FATCA Withholding Taxes
The Foreign Account Tax Compliance Act (“FATCA”), as reflected in Sections 1471 through 1474 of the Code, imposes a U.S. federal withholding tax at a rate of 30% on certain payments, including dividends paid in respect of our common stock and the gross proceeds of disposition on our common stock, made to a “foreign financial institution” (as specially defined under these rules) unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding certain U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or an exemption applies. FATCA also generally will impose a U.S. federal withholding tax of 30% on certain payments, including dividends paid in respect of our common stock and the gross proceeds of disposition on our common stock, made to a non-financial foreign entity unless such entity provides the withholding agent a certification identifying certain direct and indirect U.S. owners of the entity or an exemption applies. An intergovernmental agreement between the United States and an applicable foreign country may modify these requirements. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. FATCA currently applies to dividends paid on our common stock. Proposed Treasury Regulations, which may be relied upon until final Treasury Regulations are finalized, currently eliminate FATCA withholding on payments of gross proceeds from sales or other dispositions of our common stock.
Prospective investors are encouraged to consult with their own tax advisors regarding the possible implications of FATCA on their investment in our common stock.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAW, AS WELL AS TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL, NON-U.S. OR U.S. FEDERAL NON-INCOME TAX LAWS SUCH AS ESTATE AND GIFT TAX.
117
Subject to the terms and conditions set forth in the underwriting agreement, dated , 2022 between us and Revere Securities LLC, as representative of the underwriters named below, or the “Representative,” and the book-running managers of this offering, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the shares of common stock shown opposite its name below:
|
Underwriter |
Number of Shares |
|
|
Revere Securities LLC |
1,500,000 |
|
|
|
||
|
Total |
1,500,000 |
Under the terms of the underwriting agreement, the underwriters are committed to purchase all of the shares offered by this prospectus (other than the shares subject to the underwriters’ option to purchase additional shares), if the underwriters buy any of such shares. The underwriting agreement provides that the obligations of the several underwriters are subject to certain conditions precedent such as the receipt by the underwriters of officers’ certificates and legal opinions and approval of certain legal matters by their counsel. We have agreed to indemnify the underwriters and certain of their controlling persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make in respect of those liabilities. The underwriters have not committed to purchase and are not involved in the sale of the selling stockholder shares.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Option to Purchase Additional Shares
We have granted to the underwriters an option, exercisable for 45 days from the date of this prospectus, to purchase, from time to time, in whole or in part, up to an aggregate of 225,000 shares from us at the public offering price set forth on the cover page of this prospectus, less underwriting discounts and commissions. If the underwriters exercise this option, each underwriter will be obligated, subject to certain conditions, to purchase a number of additional shares approximately proportionate to that underwriter’s initial purchase commitment as indicated in the table above.
Commission and Expenses
The underwriters have advised us that they propose to offer the shares of common stock to the public at the public offering price set forth on the cover page of this prospectus and to certain dealers, which may include the underwriters, at that price less a concession not in excess of per share of common stock. The underwriters may allow, and certain dealers may reallow, a discount from the concession not in excess of per share of common stock to certain brokers and dealers. After the initial offering, the Representative may change the offering price and other selling terms.
We have paid the Representative a $10,000 advance upon the execution of the Letter of Engagement between us and the Representative, dated May 4, 2022 (as amended), to be credited against the accountable expenses actually incurred by the Representative in connection with this Offering. To the extent that the accountable expenses actually incurred by the Representative in connection with this Offering amount to less than $10,000, the difference will be reimbursed to us in compliance with FINRA Rule 5110(g)(4)(A).
118
Private Offering
In late November 2021 through April 2022, we sold and issued an aggregate of 3,012,280 shares of common stock to a total of 74 accredited investors at a purchase price of $2.00 per share, for an aggregate purchase price of $6,024,560. The Representative acted as placement agent in connection with such offering, and in connection therewith we agreed to pay to the Representative (i) a cash fee equal to 8.0% of the aggregate gross proceeds raised in such offering and (ii) 57,250 shares of our common stock, which shares will be issued on or after the closing of this initial public offering. The Representative has agreed not to sell, transfer, assign, pledge, or hypothecate, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of such shares for a period of 180 days following the effective date of the registration statement related to this initial public offering in accordance with FINRA Rule 5110(e)(1). However, these shares may be assigned, in whole or in part, to any officer or partner of the underwriter, and to members of the underwriting syndicate or selling group (or to officers or partners thereof). In connection with the private placement, the Representative was also given a 12 month right of first refusal which terminates upon the closing of this initial public offering.
Tail Compensation
We have agreed to pay the Representative a cash fee equal to 8.0% of the aggregate gross proceeds received by us from the sale of our common stock in any private or public offering or other financing or capital-raising transaction of any kind within the 12 month period following the effective date of the registration statement of which this prospectus is a part, provided that such financing is provided by a party actually introduced to us by the Representative.
The following table shows the public offering price, the underwriting discounts and commissions that we are to pay the underwriters and the proceeds, before expenses, to us in connection with this offering. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
|
Per Share |
Total |
|||||||||||
|
Without |
With |
Without |
With |
|||||||||
|
Public offering price |
$ |
$ |
$ |
$ |
||||||||
|
Underwriting discounts and commissions |
$ |
$ |
$ |
$ |
||||||||
|
Proceeds to us, before |
$ |
$ |
$ |
$ |
||||||||
We estimate expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $925,000. We have paid to the Representative a $10,000 advance, which will be credited against the accountable out-of-pocket expenses that are payable by us upon completion of the offering. To the extent that the accountable expenses actually incurred by the Representative in connection with this Offering amount to less than $10,000, the difference will be reimbursed to us in compliance with FINRA Rule 5110(g)(4)(A). We have agreed to reimburse the Representative up to $150,000 for their fees and expenses of legal counsel and other out-of-pocket expenses, roadshow expenses and cost of background checks. We have also agreed to reimburse the Representative for certain of their expenses incurred in connection with the offering’s settlement and closing in an amount not to exceed $12,900. Such reimbursed fees and expenses, as set forth in the underwriting agreement, are deemed underwriting compensation for this offering by FINRA.
Listing
We have applied to list our common stock on the Nasdaq Capital Market under the symbol “ASPI.” The approval of our common stock for listing on Nasdaq is a condition to the closing of this offering.
119
No Sales of Similar Securities
We, our officers and our directors have agreed, subject to certain specified exceptions, not to directly or indirectly, for a period of 12 months after the date of the underwriting agreement, in the case of us and 180 days after the date of the underwriting agreement, in the case of our officers and directors:
• sell, offer, contract or grant any option to sell (including any short sale), pledge, transfer, establish an open “put equivalent position” within the meaning of Rule 16a-l(h) under the Securities Exchange Act of 1934, as amended, or otherwise dispose of, any shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock currently or hereafter owned either of record or beneficially,
• enter into any swap, hedge or other agreement or transaction that transfers, in whole or in part, the economic consequence of ownership of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock, or
• publicly announce an intention to do any of the foregoing for a period of 180 days after the date of this prospectus without the prior written consent of the Representative.
In addition, we and each such person agrees that, without the prior written consent of the Representative, we or such other person will not, during the restricted period, make any demand for, or exercise any right with respect to, the registration of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock.
The Representative may, in their sole discretion and at any time or from time to time before the termination of the 180-day period release all or any portion of the securities subject to lock-up agreements.
Subject to compliance with the notification requirements under FINRA Rule 5131 applicable to lock-up agreements with our directors or officers, if the Representative, with our prior consent, agree to release or waive the restrictions set forth in a lock-up agreement with one of our directors or officers and provides us with notice of the impending release or waiver at least three business days before the effective date of the release or waiver, we agree to announce the impending release or waiver by a press release through a major news service at least two business days before the effective date of the release or waiver.
Market Making, Stabilization and Other Transactions
The underwriters may make a market in the common stock as permitted by applicable laws and regulations. However, the underwriters are not obligated to do so, and the underwriters may discontinue any market-making activities at any time without notice in their sole discretion. Accordingly, no assurance can be given as to the liquidity of the trading market for the common stock, that you will be able to sell any of the common stock held by you at a particular time or that the prices that you receive when you sell will be favorable.
The underwriters have advised us that they, pursuant to Regulation M under the Securities Exchange Act of 1934, as amended, and certain persons participating in the offering, may engage in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this offering. These activities may have the effect of stabilizing or maintaining the market price of the common stock at a level above that which might otherwise prevail in the open market. Establishing short sales positions may involve either “covered” short sales or “naked” short sales.
“Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of our common stock in this offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares of our common stock or purchasing shares of our common stock in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase additional shares.
“Naked” short sales are sales in excess of the option to purchase additional shares of our common stock. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of our common stock in the open market after pricing that could adversely affect investors who purchase in this offering.
120
A stabilizing bid is a bid for the purchase of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock. A syndicate covering transaction is the bid for or the purchase of shares of common stock on behalf of the underwriters to reduce a short position incurred by the underwriters in connection with the offering. Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the underwriters to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the common stock originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
Neither we, nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. The underwriters are not obligated to engage in these activities and, if commenced, may end any of these activities at any time.
Passive Market Making
The underwriters may also engage in passive market making transactions in our common stock on the NASDAQ in accordance with Rule 103 of Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specified purchase limits are exceeded. Passive market making may cause the price of our common stock to be higher than the price that otherwise would exist in the open market in the absence of those transactions. The underwriters are not required to engage in passive market making and, if commenced, may end passive market making activities at any time.
Electronic Distribution
A prospectus in electronic format may be made available by e-mail or on the web sites or through online services maintained by one or more of the underwriters, selling group members (if any) or their affiliates. The underwriters may agree with us to allocate a specific number of shares of common stock for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ web sites and any information contained in any other web site maintained by any of the underwriters is not part of this prospectus, has not been approved and/or endorsed by us or the underwriters and should not be relied upon by investors.
Other Activities and Relationships
The underwriters and certain of their respective affiliates are full service financial institutions engaged in a wide range of activities for their own accounts and the accounts of customers, which may include, among other things, corporate finance, mergers and acquisitions, merchant banking, equity and fixed income sales, trading and research, derivatives, foreign exchange, futures, asset management, custody, clearance and securities lending. The underwriters and certain of their affiliates have, from time to time, performed, and may in the future perform, various investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses.
In addition, in the ordinary course of its business, the underwriters and their respective affiliates may, directly or indirectly, hold long or short positions, trade and otherwise conduct such activities in or with respect to debt or equity securities and/or bank debt of, and/or derivative products. Such investment and securities activities may involve our securities and instruments. The underwriters and their respective affiliates may also make investment recommendations or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long or short positions in such securities and instruments.
Stamp Taxes
If you purchase shares of common stock offered in this prospectus, you may be required to pay stamp taxes and other charges under the laws and practices of the country of purchase, in addition to the offering price listed on the cover page of this prospectus.
121
Hamilton & Associates Law Group, P.A., Boca Raton, Florida will pass upon the validity of the shares of our common stock being offered by this prospectus. Carmel, Milazzo & Feil LLP, New York, New York is acting as counsel to the underwriters.
The consolidated balance sheet of ASP Isotopes Inc. and Subsidiaries as of December 31, 2021, and the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows for the period from September 13, 2021 (inception) to December 31, 2021, have been audited by EisnerAmper LLP, independent registered public accounting firm, as stated in their report which is included herein, which report includes an explanatory paragraph about the existence of substantial doubt concerning the Company’s ability to continue as a going concern. Such financial statements have been included herein in reliance on the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of our common stock offered by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, some of which is contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and our common stock, we refer you to the registration statement, including the exhibits filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or any other document are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. The SEC maintains a website that contains reports, proxy statements and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
As a result of this offering, we will become subject to the information and reporting requirements of the Exchange Act and, in accordance with this law, will file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information will be available for inspection at the website of the SEC referred to above. We also maintain a website at www.aspisotopes.com where, upon closing of this offering, you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information on or that can be accessed through our website is not a part of this prospectus and the inclusion of our website address in this prospectus is an inactive textual reference only.
122
ASP Isotopes Inc.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Page |
||
|
UNAUDITED CONDENSED FINANCIAL STATEMENTS AS OF AND FOR THE SIX-MONTH PERIOD ENDED JUNE 30, 2022 (Unaudited) |
||
|
F-2 |
||
|
Unaudited Condensed Consolidated Statement of Operations and Comprehensive Loss |
F-3 |
|
|
Unaudited Condensed Consolidated Statement of Changes in Stockholders’ Equity |
F-4 |
|
|
F-5 |
||
|
Notes to Unaudited Condensed Consolidated Financial Statements |
F-6 |
|
|
FINANCIAL STATEMENTS AS OF, AND FOR THE PERIOD FROM, SEPTEMBER 13, 2021 (INCEPTION) TO DECEMBER 31, 2021 |
||
|
F-19 |
||
|
F-20 |
||
|
F-21 |
||
|
F-22 |
||
|
F-23 |
||
|
F-24 |
F-1
ASP Isotopes Inc.
Condensed Consolidated Balance Sheets
(unaudited)
|
June 30, |
December 31, |
|||||||
|
Assets |
|
|
|
|
||||
|
Current assets: |
|
|
|
|
||||
|
Cash |
$ |
2,813,411 |
|
$ |
2,953,721 |
|
||
|
Deferred offering costs |
|
154,200 |
|
|
— |
|
||
|
Prepaid expenses and other current assets |
|
589,340 |
|
|
267,562 |
|
||
|
Total current assets |
|
3,556,951 |
|
|
3,221,283 |
|
||
|
Property and equipment, net |
|
5,576,209 |
|
|
2,988,210 |
|
||
|
Operating lease right-of-use asset |
|
893,892 |
|
|
933,145 |
|
||
|
Total assets |
$ |
10,027,052 |
|
$ |
7,142,638 |
|
||
|
|
|
|
|
|||||
|
Liabilities and stockholders’ equity |
|
|
|
|
||||
|
Current liabilities: |
|
|
|
|
||||
|
Accounts payable |
$ |
879,367 |
|
$ |
59,679 |
|
||
|
Accrued expenses |
|
619,101 |
|
|
42,500 |
|
||
|
Notes payable |
|
33,854 |
|
|
46,900 |
|
||
|
Operating lease liability – current |
|
42,412 |
|
|
38,072 |
|
||
|
Share liability |
|
240,982 |
|
|
116,200 |
|
||
|
Total current liabilities |
|
1,815,716 |
|
|
303,351 |
|
||
|
Operating lease liability – noncurrent |
|
802,798 |
|
|
841,623 |
|
||
|
Total liabilities |
|
2,618,514 |
|
|
1,144,974 |
|
||
|
Commitments and contingencies (Note 6) |
|
|
|
|
||||
|
Stockholders’ equity |
|
|
|
|
||||
|
Common stock, $0.01 par value; 50,000,000 shares authorized, 29,407,127 and 20,652,500 shares issued and outstanding at June 30, 2022 and December 31, 2021, respectively |
|
294,071 |
|
|
206,525 |
|
||
|
Additional paid-in capital |
|
11,263,158 |
|
|
8,380,343 |
|
||
|
Accumulated deficit |
|
(4,292,685 |
) |
|
(2,607,927 |
) |
||
|
Accumulated other comprehensive income |
|
143,994 |
|
|
18,723 |
|
||
|
Total stockholders’ equity |
|
7,408,538 |
|
|
5,997,664 |
|
||
|
Total liabilities and stockholders’ equity |
$ |
10,027,052 |
|
$ |
7,142,638 |
|
||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-2
ASP Isotopes Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(unaudited)
|
Six Months Ended |
||||
|
Operating expenses: |
|
|
||
|
Research and development |
$ |
446,440 |
|
|
|
General and administrative |
|
1,239,772 |
|
|
|
Total operating expenses |
|
1,686,212 |
|
|
|
Loss from operations |
|
(1,686,212 |
) |
|
|
Other income: |
|
|
||
|
Interest income |
|
1,454 |
|
|
|
Total other income |
|
1,454 |
|
|
|
Net loss |
$ |
(1,684,758 |
) |
|
|
Net loss per share, basic and diluted |
$ |
(0.06 |
) |
|
|
Weighted average shares of common stock outstanding, basic and diluted |
|
27,898,098 |
|
|
|
|
|
|||
|
Other comprehensive loss: |
|
|
||
|
Net loss |
|
(1,684,758 |
) |
|
|
Foreign currency translation |
|
125,271 |
|
|
|
Total comprehensive loss |
$ |
(1,559,487 |
) |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-3
ASP Isotopes Inc.
Condensed Consolidated Statements of Changes in Stockholders’ Equity
(unaudited)
|
Common Stock |
Additional |
Accumulated |
Accumulated |
Total |
||||||||||||||||
|
Shares |
Amount |
|||||||||||||||||||
|
Balance at December 31, 2021 |
20,652,500 |
$ |
206,525 |
$ |
8,380,343 |
|
$ |
18,723 |
$ |
(2,607,927 |
) |
$ |
5,997,664 |
|
||||||
|
Issuance of common stock, net of issuance costs totaling $380,747 |
1,559,780 |
|
15,598 |
|
2,723,215 |
|
|
— |
|
— |
|
|
2,738,813 |
|
||||||
|
Issuance of common stock upon exercise of warrants |
7,194,847 |
|
71,948 |
|
(71,948 |
) |
|
— |
|
— |
|
|
— |
|
||||||
|
Stock-based compensation |
— |
|
— |
|
231,548 |
|
|
— |
|
— |
|
|
231,548 |
|
||||||
|
Foreign currency translation |
— |
|
— |
|
— |
|
|
125,271 |
|
— |
|
|
125,271 |
|
||||||
|
Net loss |
— |
|
— |
|
— |
|
|
— |
|
(1,684,758 |
) |
|
(1,684,758 |
) |
||||||
|
Balance at June 30, 2022 |
29,407,127 |
$ |
294,071 |
$ |
11,263,158 |
|
$ |
143,994 |
$ |
(4,292,685 |
) |
$ |
7,408,538 |
|
||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-4
ASP Isotopes Inc.
Condensed Consolidated Statements of Cash Flows
(unaudited)
|
Six Months |
||||
|
Cash flows from Operating activities |
|
|
||
|
Net loss |
$ |
(1,684,758 |
) |
|
|
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
||
|
Stock-based compensation |
|
231,548 |
|
|
|
Amortization of right-of-use lease asset |
|
37,476 |
|
|
|
Changes in operating assets and liabilities: |
|
|
||
|
Deferred offering costs |
|
(154,200 |
) |
|
|
Prepaid expenses and other current assets |
|
(321,778 |
) |
|
|
Accounts payable |
|
(35,716 |
) |
|
|
Accrued expenses |
|
576,601 |
|
|
|
Lease liability |
|
(32,722 |
) |
|
|
Net cash used in operating activities |
|
(1,383,549 |
) |
|
|
Cash flows from investing activities |
|
|
||
|
Purchases of property and equipment |
|
(1,732,595 |
) |
|
|
Net cash used in investing activities |
|
(1,732,595 |
) |
|
|
Cash flows from financing Activities |
|
|
||
|
Proceeds from issuance of common stock |
|
3,119,560 |
|
|
|
Common stock issuance costs |
|
(255,965 |
) |
|
|
Repayment of notes payable |
|
(13,046 |
) |
|
|
Net cash provided by financing activities |
|
2,850,549 |
|
|
|
Net change in cash |
|
(265,595 |
) |
|
|
Effect of exchange rate changes on cash |
|
125,285 |
|
|
|
Cash – beginning of period |
|
2,953,721 |
|
|
|
Cash – end of period |
$ |
2,813,411 |
|
|
|
|
|
|||
|
Supplemental disclosures of non-cash investing and financing activities: |
|
|
||
|
Share liability for non-cash issuance costs |
$ |
124,782 |
|
|
|
Purchase of property and equipment included in accounts payable |
|
855,404 |
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-5
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
1. Organization
Description of Business
ASP Isotopes Inc. was incorporated in the state of Delaware on September 13, 2021 and has its principal operations in Boca Raton, Florida. ASP Isotopes Inc.’s subsidiary, ASP Isotopes Holdings Limited (“ASP Guernsey”), has its principal operations in Guernsey. ASP Guernsey’s subsidiary, ASP Isotopes Holdings South Africa Proprietary Limited (“ASP South Africa”), has its principal operations in South Africa. ASP Isotopes UK Ltd, a wholly owned subsidiary of the Company, was incorporated in July 2022. ASP Isotopes Inc. and its subsidiaries are collectively referred to as “the Company” throughout these consolidated statements.
The Company is an isotope enrichment company. The Company utilizes technology developed in South Africa over the past 20 years to enrich isotopes of elements or molecules with low atomic masses. Many of these elements are unsuitable for enrichment using traditional methods such as centrifuges. The Company’s first commercial product will be Molybdenum 100 (“Mo-100”), which has the potential to replace Molybdenum 99, a commonly used product in the diagnostic imaging market.
Liquidity and Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared on a basis which assumes the Company is a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from any uncertainty related to the Company’s ability to continue as a going concern. Such adjustments could be material. The Company has experienced net losses and negative cash flows from operating activities since its inception. The Company incurred net losses of $1,684,758 for the six months ended June 30, 2022 and $2,607,927 for the period from September 13, 2021 (inception) through December 31, 2021. The Company anticipates it will need to continue to raise capital through additional equity and/or debt financings and/or collaborative development agreements to fund its operations.
The Company currently expects that its cash of $2,813,411 as of June 30, 2022 will not be sufficient to fund its operating expenses and capital requirements for more than 12 months from the date the financial statements are issued. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Additional funding will be necessary to complete construction of the first enrichment facility and begin operations and although the Company has plans to seek additional funding, these plans are not currently probable.
There can be no assurance that the Company will achieve or sustain positive cash flows from operations or profitability. The Company is in the process of seeking additional equity financing. However, such funding may not be available on a timely basis on terms acceptable to the Company, or at all. If the Company is unable to raise additional capital when required or on acceptable terms, the Company may be required to further scale back or discontinue the advancement of product candidates, further reduce headcount, reorganize, merge with another entity, or cease operations.
Coronavirus Pandemic
In March 2020, the World Health Organization declared the COVID-19 outbreak a pandemic. In order to mitigate the spread of COVID-19, governments have imposed unprecedented restrictions on business operations, travel and gatherings, resulting in a global economic downturn and other adverse economic and societal impacts. The COVID-19 pandemic and its impacts continue to evolve. We cannot predict the scope and severity of disruptions as a result of COVID-19 or their impacts on us, but business disruptions for us or any of the third parties with whom we engage, including the collaborators, contract organizations, third-party manufacturers, suppliers, regulators and other third parties with whom we conduct business could materially and negatively impact our ability to conduct our business in the manner and on the timelines presently planned. The extent to which the COVID-19 pandemic may impact our business and financial performance will depend on future developments, which are highly uncertain and
F-6
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
1. Organization (cont.)
cannot be predicted with confidence, including the scope and duration of the pandemic, the extent and effectiveness of government restrictions and other actions, including relief measures, implemented to address the impact of the pandemic, and resulting economic impacts.
The actual and perceived impact of the COVID-19 pandemic is changing daily, and its ultimate effect on our business cannot be predicted. As a result, there can be no assurance that we will not experience negative impacts associated with COVID-19, which could be significant. The COVID-19 pandemic may negatively impact our business, financial condition and results of operations causing interruptions or delays in the Company’s programs and services.
2. Basis of Presentation and Summary of Significant Accounting Policies
Unaudited Financial Information
The Company’s unaudited condensed consolidated financial statements included herein have been prepared in conformity with accounting principles generally accepted in the United States of America, or GAAP, and pursuant to the rules and regulations of the Securities and Exchange Commission, or SEC. In the Company’s opinion, the information furnished reflects all adjustments, all of which are of a normal and recurring nature, necessary for a fair presentation of the financial position and results of operations for the reported interim periods. The Company considers events or transactions that occur after the balance sheet date but before the financial statements are issued to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure. The results of operations for interim periods are not necessarily indicative of results to be expected for the full year or any other interim period.
Basis of Presentation and Use of Estimates
The Company’s consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). The preparation of the Company’s consolidated financial statements requires management to make estimates and assumptions that impact the reported amounts of assets, liabilities and expenses and disclosure in the Company’s consolidated financial statements and accompanying notes. The most significant estimates in the Company’s consolidated financial statements relate to the valuation of equity instruments and estimating our accrued research and development expenses. Although these estimates are based on the Company’s knowledge of current events and actions it may undertake in the future, actual results may materially differ from these estimates and assumptions.
Principles of consolidation
The Company’s consolidated financial statements include the accounts of ASP Isotopes Inc. and its subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
Currency and currency translation
The consolidated financial statements are presented in U.S. dollars, the Company’s reporting currency. The functional currency of ASP Isotopes Inc. and ASP Guernsey is the U.S. dollar. The functional currency of the Company’s subsidiary ASP South Africa is the South African Rand. Adjustments that arise from exchange rate changes on transactions of each group entity denominated in a currency other than the functional currency are included in other income and expense in the consolidated statements of operations. Assets and liabilities of ASP South Africa are recorded in their South African Rand functional currency and translated into the U.S. dollar reporting currency of the Company at the exchange rate on the balance sheet date. Revenue, when recorded, and
F-7
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
2. Basis of Presentation and Summary of Significant Accounting Policies (cont.)
expenses of ASP South Africa are recorded in their South African Rand functional currency and translated into the U.S. dollar reporting currency of the Company at the average exchange rate prevailing during the reporting period. Resulting translation adjustments are recorded to other comprehensive income (loss).
Concentration of Credit Risk and other Risks
The Company maintains its cash in bank deposit and checking accounts that at times exceed insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant credit risk.
Cash
The Company considers all highly liquid investments with original maturities at the date of purchase of three months or less to be cash equivalents. Cash and cash equivalents are stated at fair value and may include money market funds, U.S. Treasury and U.S. government-sponsored agency securities, corporate debt, commercial paper and certificates of deposit. The Company had no cash equivalents as of June 30, 2022 and December 31, 2021.
Deferred offering costs
The Company capitalizes deferred IPO costs, which primarily consist of direct, incremental legal, professional, accounting and other third-party fees relating to the Company’s initial public offering. The deferred IPO costs will be offset against IPO proceeds upon the consummation of an offering. Should the planned IPO prove to be unsuccessful, these deferred costs, as well as additional expenses to be incurred, will be charged to operations.
Segment Information
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions. The financial information is regularly reviewed by the chief operating decision maker (“CODM”), in deciding how to allocate resources. The Company’s CODM is its chief executive officer.
Fair Value of Financial Instruments
Accounting guidance defines fair value, establishes a consistent framework for measuring fair value, and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
|
Level 1: |
Observable inputs such as quoted prices in active markets; |
|||
|
Level 2: |
Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and |
|||
|
Level 3: |
Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions. |
F-8
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
2. Basis of Presentation and Summary of Significant Accounting Policies (cont.)
The Company had a share liability (Note 9) measured at (Level 3) fair value on a recurring basis of $240,982 as of June 30, 2022. There were no transfers among Level 1, Level 2 or Level 3 categories in the six months ended June 30, 2022. The following table provides a reconciliation of the Company’s liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3):
|
Share |
|||
|
Balance, September 13, 2021 |
$ |
— |
|
|
Addition on issuance of common stock |
|
116,200 |
|
|
Balance, December 31, 2021 |
|
116,200 |
|
|
Addition on issuance of common stock |
|
124,782 |
|
|
Balance, June 30, 2022 |
$ |
240,982 |
|
The carrying amounts of accounts payable, accrued expenses and notes payable are considered to be representative of their respective fair values because of the short-term nature of those instruments.
Property and Equipment
Property and equipment include costs of assets constructed, purchased or leased under a finance lease, related delivery and installation costs and interest incurred on significant capital projects during their construction periods. Expenditures for renewals and betterments also are capitalized, but expenditures for normal repairs and maintenance are expensed as incurred. Costs associated with yearly planned major maintenance are generally deferred and amortized over 12 months or until the same major maintenance activities must be repeated, whichever is shorter. The cost and accumulated depreciation applicable to assets retired or sold are removed from the respective accounts, and gains or losses thereon are included in the statement of operations.
We assign the useful lives of our property and equipment based upon our internal engineering estimates which are reviewed periodically. The estimated useful lives of our property and equipment range from 3 to 5 years, or the shorter of the useful life or remaining life of the lease for leasehold improvements. Depreciation is recorded using the straight-line method.
Construction in progress (Note 3) is carried at cost and consists of specifically identifiable direct and indirect development and construction costs. While under construction, costs of the property are included in construction in progress until the property is placed in service, at which time costs are transferred to the appropriate property and equipment account including, but not limited to, leasehold improvements or other such accounts.
Leases
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016 02, “Leases” (“ASC 842”) establishes a right-of-use model (“ROU”) that requires a lessee to recognize a ROU asset and corresponding lease liability on the balance sheet for all leases with a term longer than 12 months. Leases will be classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the income statement as well as the reduction of the right-of-use asset. The new standard provides a number of optional practical expedients in transition. The Company has elected to apply (i) the practical expedient which allows us to not separate lease and non-lease components, for new leases and (ii) the short-term lease exemption for all leases with an original term of less than 12 months, for purposes of applying the recognition and measurements requirements in the new standard.
At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on specific facts and circumstances, the existence of an identified asset(s), if any, and the Company’s control over the use of the identified asset(s), if applicable. Operating lease liabilities and their corresponding right-of-use assets are recorded based on the present value of future lease payments over the expected lease term.
F-9
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
2. Basis of Presentation and Summary of Significant Accounting Policies (cont.)
The interest rate implicit in lease contracts is typically not readily determinable. As such, the Company will utilize the incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment.
The Company has elected to combine lease and non-lease components as a single component. Operating leases are recognized on the balance sheet as ROU lease assets, lease liabilities current and lease liabilities non-current. Fixed rents are included in the calculation of the lease balances while variable costs paid for certain operating and pass-through costs are excluded. Lease expense is recognized over the expected term on a straight-line basis.
Impairment of Long-lived Assets
Long-lived assets consist primarily of property and equipment. The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of an asset is not recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value would be assessed using a discounted cash flows or other appropriate measures of fair value. The Company did not recognize any impairment losses for the six months ended June 30, 2022.
Research and Development Costs
Research and development costs consist primarily of fees paid to consultants and facilities costs. Nonrefundable advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made. All research and development costs are expensed as incurred.
General and Administrative Costs
General and administrative expenses consist primarily of salaries and related benefits, including stock-based compensation, related to our executive, finance, business development, legal, human resources and support functions. Other general and administrative expenses include professional fees for auditing, tax, consulting and patent-related services, rent and utilities and insurance.
Stock-based Compensation
Stock-based compensation expense represents the cost of the grant date fair value of employee stock awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. As there is no active market for its common stock, the Company estimates the fair value of common stock on the date of grant based on then current facts and circumstances. Forfeitures are recognized as a reduction of stock-based compensation expense as they occur.
The Company also awards restricted stock to employees and directors. Restricted stock is generally subject to forfeiture if employment terminates prior to the completion of the vesting restrictions. The Company expenses the cost of the restricted stock, which is determined to be the fair market value of the shares of common stock underlying the restricted stock at the date of grant, ratably over the period during which the vesting restrictions lapse.
Equity-based compensation expense is classified in the statement of operations in the same manner in which the award recipients’ payroll costs are classified or in which the award recipients’ service payments are classified.
Historically, there has been no public market of the Company’s common stock. The fair value of the shares of common stock underlying the Company’s share-based awards was estimated on each grant date by the Company’s board of directors. To determine the fair value of the Company’s common stock underlying option grants, the board
F-10
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
2. Basis of Presentation and Summary of Significant Accounting Policies (cont.)
of directors considered, among other things, input from management and recent third-party financings consummated by the Company. In connection with the preparation of the financial statements for the six months ended June 30, 2022 and the period from September 13, 2021 (inception) through December 31, 2021, the Company performed a retrospective review of the fair value of its common stock related to the current events available.
Income Taxes
Deferred income tax assets and liabilities arise from temporary differences associated with differences between the financial statements and tax basis of assets and liabilities, as measured by the enacted tax rates, which are expected to be in effect when these differences reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company has generated net losses since inception and accordingly has not recorded a provision for income taxes.
The Company follows the provisions of ASC 740-10, Uncertainty in Income Taxes, or ASC 740-10. The Company has not recognized a liability for any uncertain tax positions. A reconciliation of the beginning and ending amount of unrecognized tax benefits has not been provided since there is no unrecognized benefit since the date of adoption. The Company has not recognized interest expense or penalties as a result of the implementation of ASC 740-10. If there were an unrecognized tax benefit, the Company would recognize interest accrued related to unrecognized tax benefits and penalties in income tax expense.
The Company has identified the United States, Florida, South Africa and Guernsey as its major tax jurisdictions. Refer to Note 12 for further details.
Comprehensive Loss
Comprehensive loss is defined as a change in equity during a period from transactions and other events and circumstances from non-owner sources. The Company’s comprehensive loss is comprised of net loss and the effect of currency translation adjustments.
Recently Issued Accounting Pronouncements
The Company has reviewed recently issued accounting pronouncements and plans to adopt those that are applicable to it. The Company does not expect the adoption of any recently issued pronouncements to have a material impact on its results of operations or financial position.
3. Property and Equipment
Property and equipment consist of construction in progress totaling $5,576,209 and $2,988,210 at June 30, 2022 and December 31, 2021, respectively.
The Company is currently building out the plant and office space in South Africa. All costs incurred are considered construction in progress because the work is not complete as of June 30, 2022 and December 31, 2021. There was no depreciation expense for the six months ended June 30, 2022.
F-11
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
4. Accrued Expenses
Accrued expenses consisted of accrued professional and financing fees and payroll at June 30, 2022. Accrued expenses consisted of accrued payroll at December 31, 2021.
5. Notes Payable
During 2021, the Company executed promissory notes payable with two individuals with an aggregate principal balance of approximately $46,900 (35,000 GBP). The notes were due after a period of two months followed by mutually agreed upon monthly extensions and do not bear interest. Subsequent to the issuance of the notes payable, one of the individuals became an officer of the Company.
In March 2022, one of the promissory notes totaling $13,046 (10,000 GBP) was repaid in full. As of June 30, 2022, the total promissory notes payable balance was $33,854 and have been automatically extended on a monthly basis. As of December 31, 2021, the total promissory notes payable balance was $46,900.
6. Commitments and Contingencies
Klydon Proprietary Limited
In November 2021, the Company entered into an agreement with Klydon Proprietary Limited (“Klydon”) to design and build a plant to enrich Molybdenum in South Africa. The initial phase of the project includes the building of a plant that can support the production of at least 5kgs of Mo-100, and is expected to be completed in 2022. The contracted cost for this phase is $6,800,000. The second phase of the project includes the production to be increased to 20kgs of Mo-100 with an additional cost of $6,000,000. The Company can modify the contract scope and overall costs and the contract can be cancelled by either party. As of June 30, 2022 and December 31, 2021, approximately $4,990,000 and $1,800,000, respectively, has been paid under this contract and recorded as construction in progress within property and equipment.
Two individuals who are officers and board members of Klydon received warrants to purchase common stock of the Company. See Notes 8 and 9.
Contingencies
From time to time, the Company may have certain contingent liabilities that arise in the ordinary course of its business activities. The Company accrues liabilities for such matters when future expenditures are probable and such expenditures can be reasonably estimated.
7. Lease
The Company accounts for leases in accordance with ASC 842 (Note 2). The Company is party to one operating lease in Pretoria, South Africa for office and laboratory space. The lease commenced in October 2021 with the initial term set to expire in December 2030. The Company has applied the guidance in ASC 842 and has determined that it should be classified as an operating lease. The Company’s incremental borrowing rate is approximately 7.5% based on the remaining lease term of the applicable lease. Consequently, a ROU lease asset of approximately $952,521 with a corresponding lease liability of approximately $952,521 based on the present value of the minimum rental payments of such lease was recorded at the inception of the lease. In the consolidated balance sheet at June 30, 2022, the Company has a ROU asset balance of $893,892 and a current and non-current lease liability of $42,412 and $802,798, respectively, relating to the ROU lease asset. The balance of both the ROU lease asset and the lease liabilities primarily consists of future payments under the Company’s lease in South Africa.
F-12
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
7. Lease (cont.)
Quantitative information regarding the Company’s lease for the six months ended June 30, 2022 is as follows:
|
Lease Cost |
Six Months |
|||
|
Operating lease cost |
$ |
37,476 |
|
|
|
Other Information |
|
|
||
|
Operating cash flows paid for amounts included in the measurement of lease liabilities |
$ |
50,757 |
|
|
|
Operating lease liabilities arising from obtaining right-of-use assets |
$ |
— |
|
|
|
Remaining lease term (years) |
|
8.50 |
|
|
|
Discount rate |
|
7.5 |
% |
|
Future lease payments under noncancelable leases are as follows at June 30, 2022:
|
Future Lease Payments |
Operating |
|||
|
2022 |
$ |
52,182 |
|
|
|
2023 |
|
108,278 |
|
|
|
2024 |
|
116,399 |
|
|
|
2025 |
|
125,129 |
|
|
|
2026 |
|
134,514 |
|
|
|
Thereafter |
|
646,794 |
|
|
|
Total lease payments |
$ |
1,183,296 |
|
|
|
Less: imputed interest |
|
(338,086 |
) |
|
|
Total lease liabilities |
$ |
845,210 |
|
|
|
Less current portion |
|
(42,412 |
) |
|
|
Lease liability – noncurrent |
$ |
802,798 |
|
|
Rent expense for the six months ended June 30, 2022 was $45,618.
8. License Agreements
In September 2021, the Company licensed certain intellectual property from Klydon for the development, production distribution, marketing and sale of Mo-100. The license term is 999 years, unless terminated earlier by either party under certain provisions. Any development efforts improving the intellectual property performed by either Klydon or the Company will be the property of Klydon. There are no upfront, milestone payments, nor royalties on product sales over the term of the license. Two individuals who are officers and board members of Klydon received warrants to purchase common stock of the Company. See Note 9.
In January 2022, the Company licensed certain intellectual property from Klydon for the development, production distribution, marketing and sale of uranium isotope U-235 (“U-235”). The license term is 999 years, unless terminated earlier by either party under certain provisions. Any development efforts improving the intellectual property performed by either Klydon or the Company will be the property of Klydon. The Company paid an upfront fee of $100,000, which was expensed to research and development expense. The Company is required to a nominal royalty per Kg of product sold plus 10% royalties on product net profits over the term of the contract. One of the officers, who is also a board member of Klydon, became a board member and consultant of ASP Isotopes, Inc. and an employee of ASP Guernsey in January 2022.
In July 2022, ASP Isotopes UK Ltd (a subsidiary of the Company) entered into a license agreement with Klydon, as licensor, pursuant to which ASP Isotopes UK Ltd acquired from Klydon an exclusive license to use, develop, modify, improve, subcontract and sublicense certain intellectual property rights relating to the ASP
F-13
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
8. License Agreements (cont.)
technology for the production, distribution, marketing and sale of all isotopes produced using the ASP technology (the “Klydon license agreement”). The Klydon license agreement superseded and replaced the Mo-100 license and U-235 license described in Note 8 above. The Klydon license agreement is royalty-free, has a term of 999 years and is worldwide for the development of the ASP technology and the distribution, marketing and sale of isotopes. Future production of isotopes is limited to member countries of the Nuclear Suppliers Group. In connection with the Klydon license agreement the Company agreed to make an upfront payment of $100,000 (to be included within the payments we make under the Turnkey Contract) and deferred payments of $300,000 over 24 months. Klydon has the right to terminate the exclusivity of the Klydon license agreement in the event that the licensee ceases to carry on activities related to isotope enrichment for a period longer than 24 consecutive months.
In July 2022, ASP South Africa acquired assets comprising a dormant Silicon-28 aerodynamic separation processing plant from Klydon for ZAR 6,000,000 (which at the then current exchange rate was approximately USD 354,000), which will be payable to Klydon on the later of 180 days of the acquisition and the date on which the assets generate any revenues of any nature.
9. Stockholders’ Equity
Common stock
The Company had 50,000,000 shares of common stock authorized, of which 29,407,127 shares were issued and outstanding at June 30, 2022. Common stockholders are entitled to one vote for each share of outstanding common stock held at all meetings of stockholders and written actions in lieu of meetings. Common stockholders are entitled to receive dividends for each share of outstanding common stock, if and when declared by the Board. No dividends have been declared or paid by the Company through June 30, 2022.
From September 2021 through early November 2021, the Company issued 15,100,000 shares of common stock at $0.25 per share.
From November 2021 through December 2021, the Company issued 1,452,500 shares of common stock at $2.00 per share. The Company incurred $226,000 in cash issuance costs and is required to issue 58,100 shares of common stock to the placement agent with a fair value of $116,200, which is recorded as a share liability on the balance sheet.
For the first six months of 2022, the Company issued 1,559,780 shares of common stock at $2.00 per share for gross proceeds of $3,119,560. The Company incurred $255,965 in cash issuance costs and is required to issue 62,391 shares of common stock to the placement agent with a fair value of $124,782, which is recorded as a share liability on the balance sheet.
Founder Stock
In September 2021, the Company awarded 2,000,000 shares of common stock to its founders for no cash consideration. The Company determined that the fair value of these shares was $0.25 per share and recorded stock compensation expense of $500,000 in 2021.
Common Stock Warrants
In September 2021, the Company issued warrants to purchase 7,230,822 shares of common stock at an exercise price of $0.01 per share for no cash consideration to two parties for their field of knowledge related to the technical operations of the Company. These warrants were to expire in September 2023. The Company determined that the fair value of common stock was $0.25 per share. The fair value of these warrants was determined to be $1,735,841 and was recorded as general and administrative expense.
F-14
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
9. Stockholders’ Equity (cont.)
The fair values of the warrants were estimated based on the Black-Scholes model, using the following assumptions:
|
Expected volatility |
76.5 |
% |
|
|
Weighted-average risk-free rate |
0.21 |
% |
|
|
Expected term in years |
2.00 |
|
|
|
Expected dividend yield |
0 |
% |
In January 2022, warrants to purchase 7,230,822 shares of common stock were net share settled into 7,194,847 shares of common stock per the terms of the underlying warrant agreements. No warrants were exercised in 2021.
10. Stock Compensation Plan
Equity Incentive Plan
In October 2021, the Company adopted the 2021 Stock Incentive Plan (“2021 Plan”) that provides for the issuance of common stock to employees, nonemployee directors, and consultants. Recipients of incentive stock options are eligible to purchase shares of common stock at an exercise price equal to no less than the estimated fair market value of such stock on the date of grant. The Plan provides for the grant of incentive stock options, non-statutory stock options, restricted stock, restricted stock units, stock awards and stock appreciation rights. The maximum contractual term of options granted under the Plan is ten years. The maximum number of shares initially available for issuance under the 2021 Plan was 6,000,000. As of June 30, 2022, 1,634,000 shares remain available for future grant under the Plan.
Stock Options
The following table sets forth the activity for the Company’s stock options during the periods presented:
|
Number of |
Weighted- |
Weighted |
Aggregate |
||||||||
|
Outstanding at December 31, 2021 |
400,000 |
|
$ |
0.25 |
9.8 |
$ |
700,000 |
||||
|
Granted |
2,116,000 |
|
$ |
2.00 |
|
||||||
|
Forfeited |
(250,000 |
) |
$ |
0.25 |
|
||||||
|
Outstanding at June 30, 2022 |
2,266,000 |
|
$ |
1.88 |
9.8 |
$ |
262,500 |
||||
|
|
|
|
|||||||||
|
Exercisable at June 30, 2022 |
45,833 |
|
$ |
0.73 |
9.4 |
$ |
58,333 |
||||
|
Vested or expected to vest at June 30, 2022 |
2,266,000 |
|
$ |
1.88 |
9.8 |
$ |
262,500 |
||||
The fair values of the options granted were estimated based on the Black-Scholes model, using the following assumptions:
|
Six Months |
||
|
Expected volatility |
63.0% – 64.5% |
|
|
Risk-free interest rate |
1.68% – 3.08% |
|
|
Expected term in years |
5.5 – 5.8 |
|
|
Expected dividend yield |
—% |
F-15
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
10. Stock Compensation Plan (cont.)
For the six months ended June 30, 2022, the Company granted 2,116,000 options with an exercise price of $2.00 per share, of which 288,000 options were issued to nonemployee directors that vest in April 2023 and the remaining options vest monthly over three years. The weighted average grant date fair value of options granted during 2022 was $1.17.
During 2021, the Company granted 400,000 options with an exercise price of $0.25 per share that vest monthly over three years. The weighted-average grant date fair value of options granted during 2021 was $0.15.
The Company recorded stock compensation from options of $206,548 for the six months ended June 30, 2022. As of June 30, 2022, there was $2,303,866 of unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the Plan, which is expected to be recognized over a weighted average period of approximately 2.4 years.
Stock Awards
In October 2021, the Company issued 1,500,000 shares of restricted common stock to its Chief Executive Officer. The number of shares that vest is dependent on achieving certain performance conditions and dependent market conditions upon the third anniversary from the date of grant. The Company determined that the fair value of this award was $0.25 per share for a total value of $375,000. Upon reaching the performance condition, the Company will recognize stock compensation expense over the remaining measurement period. No stock compensation was recorded for this award for the six months ended June 30, 2022.
In October 2021, the Company also issued 600,000 shares of restricted common stock to a consultant who is also a member the board of directors, that vest annually over three years. The Company determined that the fair value of this award was $0.25 per share for a total value of $150,000. Stock compensation totaling $25,000 was recorded for this award for the six months ended June 30, 2022 and $116,667 of unrecognized compensation cost related to non-vested portion is expected to be recognized over the next 2.3 years. The consulting agreement also includes potential future awards of common stock for continued service. The number of shares to be awarded will be determined on a quarterly basis of $40,000 divided by the then fair value of a share of common stock for up to eight calendar quarters following the first anniversary.
The following table summarizes vesting of restricted common stock:
|
Number of |
Weighted |
||||
|
Unvested at December 31, 2021 |
2,100,000 |
$ |
0.25 |
||
|
Vested |
— |
|
— |
||
|
Unvested at June 30 2022 |
2,100,000 |
$ |
0.25 |
||
Stock-based Compensation Expense
Stock-based compensation expense for all stock awards recognized in the accompanying consolidated statements of operations for the six months ended June 30, 2022 is as follows:
|
Six Months |
|||
|
General and administrative |
$ |
207,860 |
|
|
Research and development |
|
23,688 |
|
|
Total |
$ |
231,548 |
|
F-16
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
11. Net Loss Per Share
The Company has reported losses since inception and has computed basic net loss per share attributable to common stockholders by dividing net loss attributable to common stockholders by the weighted-average number of shares of Common Stock outstanding for the period, without consideration for potentially dilutive securities. The Company computes diluted net loss per share of Common Stock after giving consideration to all potentially dilutive shares of common stock, including options to purchase common stock and warrants to purchase common stock, outstanding during the period determined using the treasury-stock and if-converted methods, except where the effect of including such securities would be antidilutive. Because the Company has reported net losses since inception, these potential shares of Common Stock and Preferred Stock have been anti-dilutive and basic and diluted loss per share were the same for all periods presented.
The following table sets forth the computation of basic and diluted net loss per share for the six months ended June 30, 2022:
|
Six Months |
||||
|
Numerator: |
|
|
||
|
Net loss |
$ |
(1,684,758 |
) |
|
|
Denominator: |
|
|
||
|
Weighted average common stock outstanding, basic and diluted |
|
27,898,098 |
|
|
|
Net loss per share, basic and diluted |
$ |
(0.06 |
) |
|
The following table sets forth the potentially dilutive securities that have been excluded from the calculation of diluted net loss per share because to include them would be anti-dilutive (in common stock equivalent shares) at June 30, 2022:
|
Six Months |
||
|
Options to purchase common stock |
2,266,000 |
|
|
Total shares of common stock equivalents |
2,266,000 |
12. Income Taxes
The Company has no income tax expense due to operating losses incurred for the six months ended June 30, 2022. The Company has provided a full valuation allowance on the net deferred tax asset because management has determined that it is more-likely-than-not that the Company will not earn income sufficient to realize the deferred tax assets during a future period.
The Company recognizes a tax benefit from an uncertain tax position when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits. Income tax positions must meet a more likely than not recognition threshold to be recognized. The Company’s practice is to recognize interest and/or penalties related to income tax matters in income tax expense. The Company had no accrual for interest and penalties on the Company’s balance sheets and has not recognized interest and/or penalties in the statements of operations and comprehensive loss for the six months ended June 30, 2022. Uncertain tax positions are evaluated based upon the facts and circumstances that exist at each reporting period. Subsequent changes in judgment based upon new information may lead to changes in recognition, derecognition, and measurement. Adjustments may result, for example, upon resolution of an issue with the taxing authorities or expiration of a statute of limitations barring an assessment for an issue. As of June 30, 2022, there were no uncertain tax positions.
F-17
ASP Isotopes Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
For The Six Months Ended June 30, 2022
12. Income Taxes (cont.)
As of June 30, 2022, the Company did not recognize any interest and penalties associated with unrecognized tax benefits. Due to net operating losses incurred, tax years from inception remain open to examination by the Federal and State taxing jurisdictions to which we are subject. The Company is not currently under Internal Revenue Services (IRS), state or local tax examination.
Ownership changes, as defined in the IRC, may limit the amount of net operating loss carryforwards that can be utilized annually to offset future taxable income pursuant to IRC Section 382 or similar provisions. Subsequent ownership changes could further affect the limitation in future years. The Company has not completed a study to assess whether a change of control has occurred or whether there have been multiple changes of control since the Company’s formation due to the significant complexity and cost associated with such study and because there could be additional changes in control in the future. As a result, the Company is not able to estimate the effect of the change in control, if any, on the Company’s ability to utilize net operating loss and research and development credit carryforwards in the future.
13. Subsequent Events
The Company has evaluated subsequent events through September 30, 2022, the date on which the accompanying financial statements were issued and none were noted except as follows.
In July 2022, the Company issued 600,000 shares of restricted common stock (subject to quarterly vesting in equal installments over one year) to a consultant who is also a member the board of directors in connection with an amendment to the consulting agreement.
F-18
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
ASP Isotopes Inc. and Subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of ASP Isotopes Inc. and Subsidiaries (the “Company”) as of December 31, 2021, and the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity and cash flows for the period from September 13, 2021 (inception) through December 31, 2021, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2021, and the results of its operations and its cash flows for the period from September 13, 2021 (inception) through December 31, 2021 in conformity with U.S. generally accepted accounting principles.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred recurring operating losses and negative cash flows from operating activities, which raises substantial doubt about its ability to continue as a going concern. Management’s plans in regards to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ EisnerAmper LLP
We have served as the Company’s auditor since 2022.
EISNERAMPER LLP
Iselin, New Jersey
April 21, 2022
F-19
ASP Isotopes Inc.
Consolidated Balance Sheet
|
December 31, 2021 |
||||
|
Assets |
|
|
||
|
Current assets: |
|
|
||
|
Cash |
$ |
2,953,721 |
|
|
|
Prepaid expenses and other current assets |
|
267,562 |
|
|
|
Total current assets |
|
3,221,283 |
|
|
|
Property and equipment, net |
|
2,988,210 |
|
|
|
Operating lease right of use asset |
|
933,145 |
|
|
|
Total assets |
$ |
7,142,638 |
|
|
|
|
|
|||
|
Liabilities and stockholders’ equity |
|
|
||
|
Current liabilities: |
|
|
||
|
Accounts payable |
$ |
59,679 |
|
|
|
Accrued expenses |
|
42,500 |
|
|
|
Notes payable |
|
46,900 |
|
|
|
Operating lease liability – current |
|
38,072 |
|
|
|
Share liability |
|
116,200 |
|
|
|
Total current liabilities |
|
303,351 |
|
|
|
Operating lease liability – noncurrent |
|
841,623 |
|
|
|
Total liabilities |
|
1,144,974 |
|
|
|
Commitments and contingencies (Note 6) |
|
|
||
|
Stockholders’ equity |
|
|
||
|
Common stock, $0.01 par value; 50,000,000 shares authorized, 20,652,500 shares issued and outstanding at December 31, 2021 |
|
206,525 |
|
|
|
Additional paid-in capital |
|
8,380,343 |
|
|
|
Accumulated deficit |
|
(2,607,927 |
) |
|
|
Accumulated other comprehensive income |
|
18,723 |
|
|
|
Total stockholders’ equity |
|
5,997,664 |
|
|
|
Total liabilities and stockholders’ equity |
$ |
7,142,638 |
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-20
ASP Isotopes Inc.
Consolidated Statement of Operations and Comprehensive Loss
|
For The |
||||
|
Operating expenses: |
|
|
||
|
Research and development |
$ |
41,610 |
|
|
|
General and administrative |
|
2,566,432 |
|
|
|
Total operating expenses |
|
2,608,042 |
|
|
|
Loss from operations |
|
(2,608,042 |
) |
|
|
Other income: |
|
|
||
|
Interest income |
|
115 |
|
|
|
Total other income |
|
115 |
|
|
|
Loss from operations before taxes |
|
(2,607,927 |
) |
|
|
Income tax expense |
|
— |
|
|
|
Net loss |
$ |
(2,607,927 |
) |
|
|
Net loss per share, basic and diluted |
$ |
(0.16 |
) |
|
|
Weighted average shares of common stock outstanding, basic and diluted |
|
16,246,432 |
|
|
|
|
|
|||
|
Other comprehensive loss: |
|
|
||
|
Net loss |
|
(2,607,927 |
) |
|
|
Foreign currency translation |
|
18,723 |
|
|
|
Total comprehensive loss |
$ |
(2,589,204 |
) |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-21
ASP Isotopes Inc.
Consolidated Statement of Changes in Stockholders’ Equity
|
Common Stock |
||||||||||||||||||||
|
|
|
Additional Paid-in |
Other |
|
Total |
|||||||||||||||
|
Balance at September 13, 2021 (Inception) |
— |
$ |
— |
$ |
— |
|
$ |
— |
$ |
— |
|
$ |
— |
|
||||||
|
Issuance of common stock to founders |
2,000,000 |
|
20,000 |
|
480,000 |
|
|
— |
|
— |
|
|
500,000 |
|
||||||
|
Issuance of restricted common |
2,100,000 |
|
21,000 |
|
(21,000 |
) |
|
— |
|
— |
|
|
— |
|
||||||
|
Issuance of common stock, |
16,552,500 |
|
165,525 |
|
6,172,275 |
|
|
— |
|
— |
|
|
6,337,800 |
|
||||||
|
Issuance of warrants to purchase common stock |
— |
|
— |
|
1,735,841 |
|
|
— |
|
— |
|
|
1,735,841 |
|
||||||
|
Stock-based compensation |
— |
|
— |
|
13,227 |
|
|
— |
|
— |
|
|
13,227 |
|
||||||
|
Foreign currency translation |
— |
|
— |
|
— |
|
|
18,723 |
|
— |
|
|
18,723 |
|
||||||
|
Net loss |
— |
|
— |
|
— |
|
|
— |
|
(2,607,927 |
) |
|
(2,607,927 |
) |
||||||
|
Balance at December 31, 2021 |
20,652,500 |
$ |
206,525 |
$ |
8,380,343 |
|
$ |
18,723 |
$ |
(2,607,927 |
) |
$ |
5,997,664 |
|
||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-22
ASP Isotopes Inc.
Consolidated Statement of Cash Flows
|
For The |
||||
|
Cash flows from Operating activities |
|
|
||
|
Net loss |
$ |
(2,607,927 |
) |
|
|
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
||
|
Stock-based compensation |
|
13,227 |
|
|
|
Issuance of common stock to founders |
|
500,000 |
|
|
|
Issuance of warrants to purchase common stock |
|
1,735,841 |
|
|
|
Amortization of right of use lease asset |
|
19,376 |
|
|
|
Changes in operating assets and liabilities: |
|
|
||
|
Prepaid expenses and other current assets |
|
(267,562 |
) |
|
|
Accounts payable |
|
59,679 |
|
|
|
Accrued expenses |
|
42,500 |
|
|
|
Lease liability |
|
(72,826 |
) |
|
|
Net cash used in operating activities |
|
(577,692 |
) |
|
|
Cash flows from investing activities |
|
|
||
|
Purchases of property and equipment |
|
(2,988,210 |
) |
|
|
Net cash used in investing activities |
|
(2,988,210 |
) |
|
|
Cash flows from financing Activities |
|
|
||
|
Proceeds from issuance of common stock |
|
6,680,000 |
|
|
|
Common stock issuance costs |
|
(226,000 |
) |
|
|
Proceeds from issuance of notes payable |
|
46,900 |
|
|
|
Net cash provided by financing activities |
|
6,500,900 |
|
|
|
Net change in cash |
|
2,934,998 |
|
|
|
Effect of exchange rate changes on cash |
|
18,723 |
|
|
|
Cash – beginning of period |
|
— |
|
|
|
Cash – end of period |
$ |
2,953,721 |
|
|
|
|
|
|||
|
Supplemental disclosures of non-cash investing and financing activities: |
|
|
||
|
Right-of-use assets obtained in exchange for lease liability |
$ |
952,521 |
|
|
|
Share liability for non-cash issuance costs |
$ |
116,200 |
|
|
The accompanying notes are an integral part of these consolidated financial statements.
F-23
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
1. Organization
Description of Business
ASP Isotopes Inc. was incorporated in the state of Delaware on September 13, 2021 and has its principal operations in Boca Raton, Florida. ASP Isotopes Inc.’s subsidiary, ASP Isotopes Guernsey Limited (“ASP Guernsey”), has its principal operations in Guernsey. ASP Guernsey’s subsidiary, ASP Isotopes South Africa (Proprietary) Limited (“ASP South Africa”), has its principal operations in South Africa. ASP Isotopes Inc. and its subsidiaries are collectively referred to as “the Company” throughout these consolidated statements.
The Company is an isotope enrichment company. The Company utilizes technology developed in South Africa over the past 20 years to enrich isotopes of elements or molecules with low atomic masses. Many of these elements are unsuitable for enrichment using traditional methods such as centrifuges. The Company’s first commercial product will be Molybdenum 100 (“Mo-100”), which has the potential to replace Molybdenum 99, a commonly used product in the diagnostic imaging market.
Liquidity and Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared on a basis which assumes the Company is a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from any uncertainty related to the Company’s ability to continue as a going concern. Such adjustments could be material. The Company has experienced net losses and negative cash flows from operating activities since its inception. The Company has a net loss of $2,607,927 for the period from September 13, 2021 (inception) through December 31, 2021. The Company anticipates it will need to continue to raise capital through additional equity and/or debt financings and/or collaborative development agreements to fund its operations.
The Company currently expects that its cash of $2,953,721 as of December 31, 2021, together with the gross proceeds from the issuance of 1,517,605 shares of common stock for $3,035,210 from January through March 2022, will not be sufficient to fund its operating expenses and capital requirements for more than 12 months from the date the financial statements are issued. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Additional funding will be necessary to complete construction of the first enrichment facility and begin operations and although the Company has plans to seek additional funding, these plans are not currently probable.
There can be no assurance that the Company will achieve or sustain positive cash flows from operations or profitability. The Company is in the process of seeking additional equity financing. However, such funding may not be available on a timely basis on terms acceptable to the Company, or at all. If the Company is unable to raise additional capital when required or on acceptable terms, the Company may be required to further scale back or discontinue the advancement of product candidates, further reduce headcount, reorganize, merge with another entity, or cease operations.
Coronavirus Pandemic
In March 2020, the World Health Organization declared the COVID-19 outbreak a pandemic. In order to mitigate the spread of COVID-19, governments have imposed unprecedented restrictions on business operations, travel and gatherings, resulting in a global economic downturn and other adverse economic and societal impacts. The COVID-19 pandemic and its impacts continue to evolve. We cannot predict the scope and severity of disruptions as a result of COVID-19 or their impacts on us, but business disruptions for us or any of the third parties with whom we engage, including the collaborators, contract organizations, third-party manufacturers, suppliers, regulators and other third parties with whom we conduct business could materially and negatively impact our ability to conduct our business in the manner and on the timelines presently planned. The extent to which the COVID-19 pandemic may impact our business and financial performance will depend on future developments, which are highly uncertain and
F-24
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
1. Organization (cont.)
cannot be predicted with confidence, including the scope and duration of the pandemic, the extent and effectiveness of government restrictions and other actions, including relief measures, implemented to address the impact of the pandemic, and resulting economic impacts.
The actual and perceived impact of the COVID-19 pandemic is changing daily, and its ultimate effect on our business cannot be predicted. As a result, there can be no assurance that we will not experience negative impacts associated with COVID-19, which could be significant. The COVID-19 pandemic may negatively impact our business, financial condition and results of operations causing interruptions or delays in the Company’s programs and services.
2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The Company’s consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). The preparation of the Company’s consolidated financial statements requires management to make estimates and assumptions that impact the reported amounts of assets, liabilities and expenses and disclosure in the Company’s consolidated financial statements and accompanying notes. The most significant estimates in the Company’s consolidated financial statements relate to assumptions used to calculate our lease liability, the valuation of equity instruments and estimating our accrued research and development expenses. Although these estimates are based on the Company’s knowledge of current events and actions it may undertake in the future, actual results may materially differ from these estimates and assumptions.
Principles of consolidation
The Company’s consolidated financial statements for 2021 include the accounts of ASP Isotopes Inc. and its subsidiaries. All intercompany balances and transactions have been eliminated in consolidation.
Currency and currency translation
The consolidated financial statements are presented in U.S. dollars, the Company’s reporting currency. The functional currency of ASP Isotopes Inc. and ASP Guernsey is the U.S. dollar. The functional currency of the Company’s subsidiary ASP South Africa is the South African Rand. Adjustments that arise from exchange rate changes on transactions of each group entity denominated in a currency other than the functional currency are included in other income and expense in the consolidated statements of operations. Assets and liabilities of ASP South Africa are recorded in their South African Rand functional currency and translated into the U.S. dollar reporting currency of the Company at the exchange rate on the balance sheet date. Revenue, when recorded, and expenses of ASP South Africa are recorded in their South African Rand functional currency and translated into the U.S. dollar reporting currency of the Company at the average exchange rate prevailing during the reporting period. Resulting translation adjustments are recorded to other comprehensive income (loss).
Concentration of Credit Risk and other Risks
The Company maintains its cash in bank deposit and checking accounts that at times exceed insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant credit risk.
Cash
The Company considers all highly liquid investments with original maturities at the date of purchase of three months or less to be cash equivalents. Cash and cash equivalents are stated at fair value and may include money market funds, U.S. Treasury and U.S. government-sponsored agency securities, corporate debt, commercial paper and certificates of deposit. The Company had no cash equivalents as of December 31, 2021.
F-25
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
2. Basis of Presentation and Summary of Significant Accounting Policies (cont.)
Segment Information
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions. The financial information is regularly reviewed by the chief operating decision maker (“CODM”), in deciding how to allocate resources. The Company’s CODM is its chief executive officer.
Fair Value of Financial Instruments
Accounting guidance defines fair value, establishes a consistent framework for measuring fair value, and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
|
Level 1: |
Observable inputs such as quoted prices in active markets; |
|||
|
Level 2: |
Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and |
|||
|
Level 3: |
Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions. |
The carrying amounts of accounts payable, accrued expenses and notes payable are considered to be representative of their respective fair values because of the short-term nature of those instruments.
Property and Equipment
Property and equipment include costs of assets constructed, purchased or leased under a finance lease, related delivery and installation costs and interest incurred on significant capital projects during their construction periods. Expenditures for renewals and betterments also are capitalized, but expenditures for normal repairs and maintenance are expensed as incurred. Costs associated with yearly planned major maintenance are generally deferred and amortized over 12 months or until the same major maintenance activities must be repeated, whichever is shorter. The cost and accumulated depreciation applicable to assets retired or sold are removed from the respective accounts, and gains or losses thereon are included in income.
We assign the useful lives of our property and equipment based upon our internal engineering estimates which are reviewed periodically. The estimated useful lives of our property and equipment range from 3 to 5 years, or the shorter of the useful life or remaining life of the lease for leasehold improvements. Depreciation is recorded using the straight-line method.
Construction in progress (Note 3) is carried at cost and consists of specifically identifiable direct and indirect development and construction costs. While under construction, costs of the property are included in construction in progress until the property is placed in service, at which time costs are transferred to the appropriate property and equipment account including, but not limited to, leasehold improvements or other such accounts.
Leases
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016 02, “Leases” (“ASC 842”) establishes a right-of-use model (“ROU”) that requires a lessee to recognize a ROU asset and corresponding lease liability on the balance sheet for all leases with a term longer than 12 months. Leases will be classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the income statement as well as the reduction of the right of use asset.
F-26
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
2. Basis of Presentation and Summary of Significant Accounting Policies (cont.)
The new standard provides a number of optional practical expedients in transition. The Company has elected to apply (i) the practical expedient which allows us to not separate lease and non-lease components, for new leases and (ii) the short-term lease exemption for all leases with an original term of less than 12 months, for purposes of applying the recognition and measurements requirements in the new standard.
At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on specific facts and circumstances, the existence of an identified asset(s), if any, and the Company’s control over the use of the identified asset(s), if applicable. Operating lease liabilities and their corresponding right-of-use assets are recorded based on the present value of future lease payments over the expected lease term. The interest rate implicit in lease contracts is typically not readily determinable. As such, the Company will utilize the incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment.
The Company has elected to combine lease and non-lease components as a single component. Operating leases are recognized on the balance sheet as ROU lease assets, lease liabilities current and lease liabilities non-current. Fixed rents are included in the calculation of the lease balances while variable costs paid for certain operating and pass-through costs are excluded. Lease expense is recognized over the expected term on a straight-line basis.
Impairment of Long-lived Assets
Long-lived assets consist primarily of property and equipment. The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of an asset is not recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value would be assessed using a discounted cash flows or other appropriate measures of fair value. The Company did not recognize any impairment losses for the period from September 13, 2021 (inception) through December 31, 2021.
Research and Development Costs
Research and development costs consist primarily of fees paid to consultants and facilities costs. Nonrefundable advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made. All research and development costs are expensed as incurred.
General and Administrative Costs
General and administrative expenses consist primarily of salaries and related benefits, including stock-based compensation, related to our executive, finance, business development, legal, human resources and support functions. Other general and administrative expenses include professional fees for auditing, tax, consulting and patent-related services, rent and utilities and insurance.
Stock-based Compensation
Stock-based compensation expense represents the cost of the grant date fair value of employee stock awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. As there is no active market for its common stock, the Company estimates the fair value of common stock on the date of grant based on then current facts and circumstances. Forfeitures are recognized as a reduction of stock-based compensation expense as they occur.
F-27
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
2. Basis of Presentation and Summary of Significant Accounting Policies (cont.)
The Company also awards restricted stock to employees and directors. Restricted stock is generally subject to forfeiture if employment terminates prior to the completion of the vesting restrictions. The Company expenses the cost of the restricted stock, which is determined to be the fair market value of the shares of common stock underlying the restricted stock at the date of grant, ratably over the period during which the vesting restrictions lapse.
Equity-based compensation expense is classified in the statement of operations in the same manner in which the award recipients’ payroll costs are classified or in which the award recipients’ service payments are classified.
Historically, there has been no public market of the Company’s common stock. The fair value of the shares of common stock underlying the Company’s share-based awards was estimated on each grant date by the Company’s board of directors. To determine the fair value of the Company’s common stock underlying option grants, the board of directors considered, among other things, input from management and recent third-party financings consummated by the Company. In connection with the preparation of the financial statements for the period from September 13, 2021 (inception) through December 31, 2021, the Company performed a retrospective review of the fair value of its common stock related to the current events available. Based on this review, the Company recorded stock compensation as reflected in the financial statements.
Income Taxes
Deferred income tax assets and liabilities arise from temporary differences associated with differences between the financial statements and tax basis of assets and liabilities, as measured by the enacted tax rates, which are expected to be in effect when these differences reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company has generated net losses since inception and accordingly has not recorded a provision for income taxes.
The Company follows the provisions of ASC 740-10, Uncertainty in Income Taxes, or ASC 740-10. The Company has not recognized a liability for any uncertain tax positions. A reconciliation of the beginning and ending amount of unrecognized tax benefits has not been provided since there is no unrecognized benefit since the date of adoption. The Company has not recognized interest expense or penalties as a result of the implementation of ASC 740-10. If there were an unrecognized tax benefit, the Company would recognize interest accrued related to unrecognized tax benefits and penalties in income tax expense.
The Company has identified the United States, South Africa and Guernsey as its major tax jurisdictions. Refer to Note 12 for further details.
Comprehensive Loss
Comprehensive loss is defined as a change in equity during a period from transactions and other events and circumstances from non-owner sources. The Company’s comprehensive loss is comprised of currency translation.
Recently Issued Accounting Pronouncements
The Company has reviewed recently issued accounting pronouncements and plans to adopt those that are applicable to it. The Company does not expect the adoption of any recently issued pronouncements to have an impact on its results of operations or financial position.
F-28
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
3. Property and Equipment
Property and equipment consist of construction in progress totaling $2,988,210 at December 31, 2021.
The Company is currently building out the plant and office space in South Africa. All costs incurred are considered construction in progress because the work is not complete as of December 31, 2021. There was no depreciation expense for the period from September 13, 2021 (inception) through December 31, 2021.
4. Accrued Expenses
Accrued expenses consisted of accrued payroll at December 31, 2021.
5. Notes Payable
During 2021, the Company executed promissory notes payable with two individuals with an aggregate principal balance of approximately $46,900 (35,000 GBP). The notes were due after a period of two months followed by mutually agreed upon monthly extensions and do not bear interest. Subsequent to the issuance of the notes payable, one of the individuals became an officer of the Company. As of December 31, 2021, the total promissory notes payable balance was $46,900 and have been automatically extended.
6. Commitments and Contingencies
Klydon Proprietary Limited
In November 2021, the Company entered into an agreement with Klydon Proprietary Limited (“Klydon”) to design and build a plant to enrich Molybdenum in South Africa. The initial phase of the project includes the building of a plant that can support the production of at least 5kgs of Mo-100, and is expected to be completed in 2022. The contracted cost for this phase is $6,800,000. The second phase of the project includes the production to be increased to 20kgs of Mo-100 with an additional cost of $6,000,000. The Company can modify the contract scope and overall costs and the contract can be cancelled by either party. As of December 31, 2021, approximately $1,800,000 has been paid under this contract and recorded as construction in progress.
In September 2021, the Company issued warrants to purchase common stock of the Company to two individuals who are officers and board members of Klydon. See Note 9.
Contingencies
From time to time, the Company may have certain contingent liabilities that arise in the ordinary course of its business activities. The Company accrues liabilities for such matters when future expenditures are probable and such expenditures can be reasonably estimated.
7. Lease
The Company accounts for leases in accordance with ASC 842 (Note 2). The Company is party to one operating lease in Pretoria, South Africa for office and laboratory space. The lease commenced in October 2021 with the initial term set to expire in December 2030. The Company has applied the guidance in ASC 842 and has determined that it should be classified as an operating lease. The Company’s incremental borrowing rate is approximately 7.5% based on the remaining lease term of the applicable lease. Consequently, a ROU lease asset of approximately $952,521 with a corresponding lease liability of approximately $952,521 based on the present value of the minimum rental payments of such lease was recorded at the inception of the lease. In the consolidated balance sheet at December 31, 2021, the Company has a ROU asset balance of $933,145 and a current and non-current lease liability of $38,072 and $841,623, respectively, relating to the ROU lease asset. The balance of both the ROU lease asset and the lease liabilities primarily consists of future payments under the Company’s lease in South Africa.
F-29
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
7. Lease (cont.)
Quantitative information regarding the Company’s lease for the period September 13, 2021 (inception) through December 31, 2021 is as follows:
|
Lease Cost |
For the |
|||
|
Operating lease cost |
$ |
19,376 |
|
|
|
Other Information |
|
|
||
|
Operating cash flows paid for amounts included in the measurement of lease liabilities |
$ |
26,582 |
|
|
|
Operating lease liabilities arising from obtaining right-of-use assets |
$ |
952,521 |
|
|
|
Remaining lease term (years) |
|
9.00 |
|
|
|
Discount rate |
|
7.5 |
% |
|
Future lease payments under noncancelable leases are as follows at December 31, 2021:
|
Operating |
||||
|
Future Lease Payments |
|
|
||
|
2022 |
$ |
102,828 |
|
|
|
2023 |
|
110,540 |
|
|
|
2024 |
|
118,831 |
|
|
|
2025 |
|
127,743 |
|
|
|
2026 |
|
137,324 |
|
|
|
Thereafter |
|
660,305 |
|
|
|
Total lease payments |
$ |
1,257,571 |
|
|
|
Less: imputed interest |
|
(377,876 |
) |
|
|
Total lease liabilities |
$ |
879,695 |
|
|
|
Less current portion |
|
(38,072 |
) |
|
|
Lease liability – noncurrent |
|
841,623 |
|
|
Rent expense for the period September 13, 2021 (inception) through December 31, 2021 was $36,366.
8. License Agreements
In September 2021, the Company licensed certain intellectual property from Klydon for the development, production distribution, marketing and sale of Mo-100. The license term is 999 years, unless terminated earlier by either party under certain provisions. Any development efforts improving the intellectual property performed by either Klydon or the Company will be the property of Klydon, however any additions to know how or improvements to the technology will be deemed part of the intellectual property rights licensed to the Company under the Mo-100 license. There are no upfront, milestone payments, nor royalties on product sales over the term of the license.
In September 2021, the Company issued warrants to purchase common stock of the Company to two individuals who are officers and board members of Klydon. See Note 9.
F-30
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
8. License Agreements (cont.)
In January 2022, the Company licensed certain intellectual property from Klydon for the development, production distribution, marketing and sale of uranium isotope U-235 (“U-235”). The license term is 999 years, unless terminated earlier by either party under certain provisions. Any development efforts improving the intellectual property performed by either Klydon or the Company will be the property of Klydon, however any additions to know how or improvements to the technology will be deemed part of the intellectual property rights licensed to the Company under the U-235 license. The Company paid an upfront fee of $100,000, which will be expensed to research and development expense. The Company is required to a nominal royalty per Kg of product sold plus 10% royalties on product net profits over the term of the contract.
One individual who is an officer and a director of Klydon became a director and consultant of the Company and an employee of ASP Guernsey in January 2022. Another individual who is an officer and a director of Klydon became an advisor to the Company in January 2022.
9. Stockholders’ Equity
Common stock
The Company had 50,000,000 shares of common stock authorized, of which 20,652,500 shares were issued and outstanding at December 31, 2021. Common stockholders are entitled to one vote for each share of outstanding common stock held at all meetings of stockholders and written actions in lieu of meetings. Common stockholders are entitled to receive dividends for each share of outstanding common stock, if and when declared by the Board. No dividends have been declared or paid by the Company through December 31, 2021.
From September 2021 through early November 2021, the Company issued 15,100,000 shares of common stock at $0.25 per share.
From November 2021 through December 2021, the Company issued 1,452,500 shares of common stock at $2.00 per share. The Company incurred $226,000 in cash issuance costs and is required to issue 58,100 shares of common stock to the placement agent with a fair value of $116,200, which is recorded as a share liability on the balance sheet.
Subsequent to December 31, 2021, the Company has issued 1,517,605 shares of common stock for gross proceeds of $3,035,210.
Founder Stock
In September 2021, the Company awarded 2,000,000 shares of common stock to its founders for no cash consideration. The Company determined that the fair value of these shares was $0.25 per share and recorded stock compensation expense of $500,000 in 2021.
Common Stock Warrants
In September 2021, the Company issued warrants to purchase 7,230,822 shares of common stock at an exercise price of $0.01 per share for no cash consideration to two parties for their field of knowledge related to the technical operations of the Company. These warrants expire in September 2023. The Company determined that the fair value of the common shares was $0.25 per share. The fair value of these warrants was determined to be $1,735,841 and was recorded as general and administrative expense. No warrants were exercised in 2021.
F-31
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
9. Stockholders’ Equity (cont.)
The fair values of the warrants were estimated based on the Black-Scholes model, using the following assumptions:
|
Expected volatility |
76.5 |
% |
|
|
Weighted-average risk-free rate |
0.21 |
% |
|
|
Expected term in years |
2.00 |
|
|
|
Expected dividend yield |
— |
% |
In January 2022, warrants to purchase 7,230,822 shares of common stock were net share settled into 7,194,847 shares of common stock per the terms of the underlying warrant agreements.
10. Stock Compensation Plan
Equity Incentive Plan
In October 2021, the Company adopted the 2021 Stock Incentive Plan (“2021 Plan”) that provides for the issuance of common stock to employees, nonemployee directors, and consultants. Recipients of incentive stock options are eligible to purchase shares of common stock at an exercise price equal to no less than the estimated fair market value of such stock on the date of grant. The Plan provides for the grant of incentive stock options, non-statutory stock options, restricted stock, restricted stock units, stock awards and stock appreciation rights. The maximum contractual term of options granted under the Plan is ten years. As of December 31, 2021, the maximum number of shares initially available for issuance under the 2021 Plan was 6,000,000. As of December 31, 2021, 3,500,000 shares remain available for future grant under the Plan.
Stock Options
The following table sets forth the activity for the Company’s stock options during the periods presented:
|
|
|
Weighted |
|
|||||||
|
Outstanding at September 13, 2021 |
— |
$ |
— |
— |
$ |
— |
||||
|
Granted |
400,000 |
$ |
0.25 |
|
||||||
|
Outstanding at December 31, 2021 |
400,000 |
$ |
0.25 |
9.8 |
$ |
700,000 |
||||
|
|
|
|||||||||
|
Exercisable at December 31, 2021 |
22,222 |
$ |
0.25 |
9.8 |
$ |
38,889 |
||||
|
Vested or expected to vest at December 31, 2021 |
400,000 |
$ |
0.25 |
9.8 |
$ |
700,000 |
||||
The fair values of the options granted were estimated based on the Black-Scholes model, using the following assumptions:
|
2021 |
|||
|
Expected volatility |
69.5 |
% |
|
|
Weighted-average risk-free rate |
1.11 |
% |
|
|
Expected term in years |
5.77 |
|
|
|
Expected dividend yield |
— |
% |
|
During 2021, the Company granted 400,000 options with an exercise price of $0.25 per share that vest monthly over three years. The weighted-average grant date fair value of options granted during 2021 was $0.15. The Company recorded stock compensation from options of $4,894 for the period from September 13, 2021 (inception)
F-32
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
10. Stock Compensation Plan (cont.)
through December 31, 2021. As of December 31, 2021, there was $56,004 of unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the Plan, which is expected to be recognized over a weighted average period of approximately 2.8 years.
Stock Awards
In October 2021, the Company issued 1,500,000 shares of restricted common stock to its Chief Executive Officer. The number of shares that vest is dependent on achieving certain performance conditions and dependent market conditions upon the third anniversary from the date of grant. The Company determined that the fair value of this award was $0.25 per share for a total value of $375,000. Upon reaching the performance condition, the Company will recognize stock compensation expense over the remaining measurement period. No stock compensation was recorded for this award in 2021.
In October 2021, the Company also issued 600,000 shares of restricted common stock to a consultant who is also a member the board of directors, that vest annually over three years. The Company determined that the fair value of this award was $0.25 per share for a total value of $150,000. Stock compensation totaling $8,333 was recorded for this award in 2021 and $141,667 of unrecognized compensation cost related to non-vested portion is expected to be recognized over the next 2.8 years. The consulting agreement also includes potential future awards of common stock for continued service. The number of shares to be awarded will be determined on a quarterly basis of $40,000 divided by the then fair value of a share of common stock for up to eight calendar quarters following the first anniversary.
The following table summarizes vesting of restricted common stock:
|
|
Weighted |
||||
|
Unvested as of September 13, 2021 (inception) |
— |
$ |
— |
||
|
Issuance of restricted common stock |
2,100,000 |
$ |
0.25 |
||
|
Unvested at December 31, 2021 |
2,100,000 |
$ |
0.25 |
||
Stock-based Compensation Expense
Stock-based compensation expense for all stock awards recognized in the accompanying consolidated statements of operations for the period September 13, 2021 (inception) through December 31, 2021 is as follows:
|
2021 |
|||
|
General and administrative |
$ |
513,227 |
|
|
Total |
$ |
513,227 |
|
11. Net Loss Per Share
The Company has reported losses since inception and has computed basic net loss per share attributable to common stockholders by dividing net loss attributable to common stockholders by the weighted-average number of shares of Common Stock outstanding for the period, without consideration for potentially dilutive securities. The Company computes diluted net loss per share of Common Stock after giving consideration to all potentially dilutive shares of common stock, including options to purchase common stock and warrants to purchase common stock, outstanding during the period determined using the treasury-stock and if-converted methods, except where the effect of including such securities would be antidilutive. Because the Company has reported net losses since inception, these potential shares of Common Stock and Preferred Stock have been anti-dilutive and basic and diluted loss per share were the same for all periods presented.
F-33
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
11. Net Loss Per Share (cont.)
The following table sets forth the computation of basic and diluted net loss per share for the period September 13, 2021 (inception) through December 31, 2021:
|
2021 |
||||
|
Numerator: |
|
|
||
|
Net loss |
$ |
(2,607,927 |
) |
|
|
Denominator: |
|
|
||
|
Weighted average common stock outstanding, basic and diluted |
|
16,246,432 |
|
|
|
Net loss per share, basic and diluted |
$ |
(0.16 |
) |
|
The following table sets forth the potentially dilutive securities that have been excluded from the calculation of diluted net loss per share because to include them would be anti-dilutive (in common stock equivalent shares) at December 31, 2021:
|
2021 |
||
|
Options to purchase common stock |
400,000 |
|
|
Warrants to purchase Common Stock |
7,230,822 |
|
|
Total shares of common stock equivalents |
7,630,822 |
12. Income Taxes
The effective tax rate of the Company’s provision (benefit) for income taxes differs from the federal statutory rate for the period September 13, 2021 (inception) through December 31, 2021 as follows:
|
2021 |
||||
|
Tax computed at federal statutory rate |
$ |
21.00 |
% |
|
|
Earnings in jurisdictions taxed at rates different from the statutory U.S. federal tax rate |
|
4.11 |
% |
|
|
Permanent differences |
|
(22.95 |
)% |
|
|
Valuation allowance |
|
(2.16 |
)% |
|
|
Income tax expense |
$ |
— |
|
|
Deferred income taxes reflect the net tax effects of (a) temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes, and (b) operating losses and tax credit carryforwards. Significant components of deferred tax assets (liabilities) at December 31, 2021 are as follows:
|
December 31, 2021 |
||||
|
Deferred tax assets: |
|
|
||
|
Net operating loss carryforwards |
$ |
140,241 |
|
|
|
Right-of-use lease liability |
|
264,249 |
|
|
|
Total deferred tax assets |
|
404,490 |
|
|
|
|
|
|||
|
Deferred tax liabilities: |
|
|
||
|
Share-based compensation |
|
(91,691 |
) |
|
|
Right-of-use lease asset |
|
(261,281 |
) |
|
|
Total deferred tax liabilities |
|
(352,972 |
) |
|
|
Total net deferred tax assets |
|
51,518 |
|
|
|
Less: valuation allowance |
|
(51,518 |
) |
|
|
Net deferred taxes |
$ |
— |
|
|
F-34
ASP Isotopes Inc.
Notes to Consolidated Financial Statements
For The Period September 13, 2021 (Inception) Through December 31, 2021
12. Income Taxes (cont.)
The Company provided a full valuation allowance on the net deferred tax asset because management has determined that it is more-likely-than-not that the Company will not earn income sufficient to realize the deferred tax assets during the carryforward period. As of December 31, 2021, the Company has federal, state and South Africa NOLs available of approximately $514,562, $514,562 and 99,139, respectively, to offset future taxable income, if any, for federal and state income tax purposes. The state NOLs are carried forward indefinitely until used and never expire. Under the Tax Act, federal NOLs utilized are limited to 80% of taxable income in any year where taxable income is determined without regard to the NOL deduction itself. The Tax Act generally eliminates the ability to carry back any net operating loss to prior taxable years, while allowing unused net operating losses to be carried forward indefinitely.
The Company recognizes a tax benefit from an uncertain tax position when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits. Income tax positions must meet a more likely than not recognition threshold to be recognized. The Company’s practice is to recognize interest and/or penalties related to income tax matters in income tax expense. The Company had no accrual for interest and penalties on the Company’s balance sheets and has not recognized interest and/or penalties in the statements of operations and comprehensive loss for the period from September 13, 2021 (inception) through December 31, 2021. Uncertain tax positions are evaluated based upon the facts and circumstances that exist at each reporting period. Subsequent changes in judgment based upon new information may lead to changes in recognition, derecognition, and measurement. Adjustments may result, for example, upon resolution of an issue with the taxing authorities or expiration of a statute of limitations barring an assessment for an issue. As of December 31, 2021, there were no uncertain tax positions.
As of December 31, 2021, the Company did not recognize any interest and penalties associated with unrecognized tax benefits. Due to net operating losses incurred, tax years from inception remain open to examination by the Federal and State taxing jurisdictions to which we are subject. The Company is not currently under Internal Revenue Services (IRS), state or local tax examination.
Ownership changes, as defined in the IRC, may limit the amount of net operating loss carryforwards that can be utilized annually to offset future taxable income pursuant to IRC Section 382 or similar provisions. Subsequent ownership changes could further affect the limitation in future years. The Company has not completed a study to assess whether a change of control has occurred or whether there have been multiple changes of control since the Company’s formation due to the significant complexity and cost associated with such study and because there could be additional changes in control in the future. As a result, the Company is not able to estimate the effect of the change in control, if any, on the Company’s ability to utilize net operating loss and research and development credit carryforwards in the future.
13. Subsequent Events
The Company has evaluated subsequent events through April 21, 2022, the date on which the accompanying financial statements were issued and none were noted except as previously disclosed in Note 8 and Note 9.
F-35
Shares
ASP Isotopes Inc.
Common Stock
Sole Book Running Manager
Revere Securities LLC
Through and including _________, 2022 (the 25th day after the date of this prospectus), all dealers effecting transactions in the common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table sets forth all expenses to be paid by ASP Isotopes Inc. (the “Registrant”), incurred or to be incurred in connection with this offering, which are inclusive of the fees and expenses incidental to the registration of the selling stockholder shares. All amounts shown are estimates except for the SEC registration fee and the FINRA filing fee.
|
SEC registration fee |
$ |
1,500.00 |
|
|
FINRA filing fee |
|
2,920.00 |
|
|
Exchange listing fee |
|
75,000.00 |
|
|
Printing and engraving expenses |
|
75,000.00 |
|
|
Legal fees and expenses |
|
550,000.00 |
|
|
Accounting fees and expenses |
|
190,000.00 |
|
|
Transfer agent and registrar fees |
|
6,500.00 |
|
|
Miscellaneous expenses |
|
24,080.00 |
|
|
Total |
$ |
925,000.00 |
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act. The Registrant’s amended and restated certificate of incorporation permits the Registrant to indemnify its directors, officers, employees and other agents to the maximum extent permitted by the Delaware General Corporation Law, and the Registrant’s amended and restated bylaws that will be in effect upon the closing of this offering provide that the Registrant will indemnify its directors and officers and permit the Registrant to indemnify its employees and other agents, in each case to the maximum extent permitted by the Delaware General Corporation Law.
The Registrant has entered into indemnification agreements with its directors and officers, whereby it have agreed to indemnify its directors and officers to the fullest extent permitted by law, including indemnification against expenses and liabilities incurred in legal proceedings to which the director or officer was, or is threatened to be made, a party by reason of the fact that such director or officer is or was a director, officer, employee or agent of the Registrant, provided that such director or officer acted in good faith and in a manner that the director or officer reasonably believed to be in, or not opposed to, the best interest of the Registrant. At present, there is no pending litigation or proceeding involving a director or officer of the Registrant regarding which indemnification is sought, nor is the registrant aware of any threatened litigation that may result in claims for indemnification.
The Registrant maintains insurance policies that indemnify its directors and officers against various liabilities arising under the Securities Act and the Exchange Act that might be incurred by any director or officer in his or her capacity as such.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
Set forth below is information regarding unregistered securities issued by us since our inception on September 13, 2021. Also included is the consideration received by us for such securities and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed. None of the following transactions involved any underwriters, underwriting discounts or commissions, or any public offering, except as noted in paragraph 6 below.
1. On September 13, 2021, we closed stock purchase agreements with our founders to issue an aggregate of 2,000,000 shares of common stock in consideration for the purchasers’ transfer to the company of all of the purchaser’s rights in certain business concepts and technology.
II-1
2. On September 15, 2021, we issued two consultants each a warrant to purchase 3,615,411 shares of common stock, with an exercise price per share of $0.01 and a term of two (2) years, for services. On January 28, 2022, the holders of the warrants exercised their warrants and the company issued an aggregate of 7,194,847 shares of common stock.
3. In late September 2021, we sold and issued an aggregate of 8,300,000 shares of common stock to a total of 9 accredited investors at a purchase price of $0.25 per share, for an aggregate purchase price of $2,075,000.
4. In October 2021 through early November 2021, we sold and issued an aggregate of 6,800,000 shares of common stock to a total of 14 accredited investors at a purchase price of $0.25 per share, for an aggregate purchase price of $1,700,000.
5. In October 2021, we sold and issued an aggregate of 1,500,000 shares of our common stock pursuant to a performance share award grant notice to Paul Mann, our Chairman, Chief Executive Officer and director, as consideration for his services to us. In addition, in October 2021, we sold and issued 600,000 shares of our common stock pursuant to a restricted stock award grant notice to a consultant (an entity owned by Sergey Vasnetsov, our director), as consideration for services to us as contemplated by the advisory agreement with us.
6. In late November 2021 through April 2022, we sold and issued an aggregate of 3,012,280 shares of common stock to a total of 74 accredited investors at a purchase price of $2.00 per share, for an aggregate purchase price of $6,024,560. Revere Securities LLC acted as placement agent in connection with such offering of shares of our common stock and in connection therewith we agreed to pay to Revere Securities LLC (i) a cash fee equal to 8.0% of the aggregate gross proceeds raised in such offering and (ii) 57,250 shares of our common stock.
7. In July 2022, we sold and issued (i) 600,000 shares of our common stock pursuant to a restricted stock award grant notice to a consultant (an entity owned by Sergey Vasnetsov, our director), as consideration for services to us as contemplated by the advisory agreement (as amended) with us and (ii) 100,000 shares of our common stock pursuant to a restricted stock award grant notice to a consultant as consideration for services to us.
8. In October 2022, we issued to our executive officers, directors and consultants 3,000,000 shares of restricted stock that we anticipate making upon the effectiveness of the registration statement of which this prospectus is a part. All of these awards are contingent upon completion of this offering.
9. From September 13, 2021 to the effective date of this registration statement, we granted stock options under our 2021 equity incentive plan, as amended (the Prior Plan), to purchase up to an aggregate of 3,151,000 shares of our common stock to our employees, directors and consultants, at a weighted-average exercise price of $1.78 per share. Through the effective date of this registration statement, no shares of common stock were issued upon the exercise of options granted to employees, directors and consultants.
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or our public offering, except as noted in paragraph 6 above. Except as described in the following paragraph, we believe that the transactions described above were exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act (and Regulation D promulgated thereunder). The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof. The sales of these securities were made without any general solicitation or advertising.
The offers, sales and issuances of the securities described in paragraphs (2), (5), (7) and (8) were deemed to be exempt from registration under the Securities Act in reliance on either Rule 701 in that the transactions were under compensatory benefit plans and contracts relating to compensation as provided under Rule 701 or Section 4(a)(2) in that the issuance of securities to the accredited investors did not involve a public offering. The recipients of such securities were our employees, directors or bona fide consultants and received the securities under the Prior Plan.
II-2
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
(a) Exhibits.
|
Exhibit |
Exhibit Description |
|
|
1.1* |
||
|
3.1* |
||
|
3.2* |
||
|
3.3* |
||
|
3.4* |
Amended and Restated Bylaws, to be effective immediately prior to closing of this offering. |
|
|
4.1*+ |
||
|
5.1* |
||
|
10.1*+ |
ASP Isotopes Inc. 2021 Stock Incentive Plan, as amended, and form of award agreements thereunder. |
|
|
10.2*+ |
ASP Isotopes Inc. 2022 Equity Incentive Plan and form of award agreements thereunder. |
|
|
10.3*+ |
||
|
10.4*+ |
||
|
10.5*+ |
||
|
10.6*+ |
Executive Employment Agreement by and between the registrant and Paul Mann, dated October 4, 2021. |
|
|
10.7*+ |
||
|
10.8*+ |
||
|
10.9* |
||
|
10.10* |
||
|
10.11* |
||
|
10.12* |
||
|
10.13* |
||
|
10.14* |
Chief Scientific Adviser between the registrant and Dr Einar Ronander, dated January 2021 |
|
|
10.15* |
||
|
10.16* |
||
|
10.17* |
License Agreement between ASP Isotopes UK Ltd and Klydon (Proprietary) Limited dated July 26, 2022. |
|
|
21.1* |
||
|
23.1 |
Consent of EisnerAmper LLP, independent registered public accounting firm. |
|
|
23.2* |
Consent of Hamilton & Associates Law Group, P.A. (included in Exhibit 5.1). |
|
|
24.1* |
||
|
107* |
____________
* Previously filed.
+ Management contract or compensatory plan or arrangement.
(b) Financial Statement Schedules. All financial statement schedules are omitted because the information called for is not required or is shown either in the consolidated financial statements or in the notes thereto.
(c) Filing Fee Table. The Filing Fee Table and related disclosure is filed herewith as Exhibit 107.
II-3
ITEM 17. UNDERTAKINGS.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933, as amended (the “Securities Act”);
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Securities and Exchange Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser:
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B) Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
II-4
(5) That for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
Insofar as indemnification for liabilities arising under the Securities Act, may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Amendment No. 5 to the registration statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in Boca Raton, Florida, on the 8th day of November, 2022.
|
ASP Isotopes Inc. |
||||
|
By: |
/s/ Paul E. Mann |
|||
|
Paul E. Mann |
||||
Pursuant to the requirements of the Securities Act of 1933, as amended, this Amendment No. 5 to the registration statement on Form S-1 has been signed by the following persons in the capacities and on the dates indicated.
|
Signature |
Title |
Date |
||
|
/s/ Paul E. Mann |
Chairman, Chief Executive Officer and |
November 8, 2022 |
||
|
Paul E. Mann |
Director (Principal Executive Officer) |
|||
|
/s/ Robert Ainscow |
Interim Chief Financial Officer |
November 8, 2022 |
||
|
Robert Ainscow |
(Principal Financial and Accounting Officer) |
|||
|
* |
Director |
November 8, 2022 |
||
|
Joshua Donfeld |
||||
|
* |
Director |
November 8, 2022 |
||
|
Duncan Moore, Ph.D. |
||||
|
* |
Director |
November 8, 2022 |
||
|
Hendrik Strydom, Ph.D. |
||||
|
* |
Director |
November 8, 2022 |
||
|
Sergey Vasnetsov |
||||
|
* |
Director |
November 8, 2022 |
||
|
Todd Wider, M.D. |
|
*By: |
/s/ Paul E. Mann |
|||
|
Paul E. Mann |
||||
|
Attorney-in-Fact |
II-6